Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-11-11 , DOI: 10.1016/j.bmcl.2017.10.057 David S. Huang , Henry L. Wong , Gunda I. Georg
|
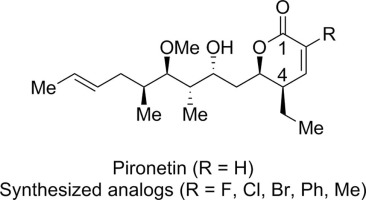
Pironetin is an α-tubulin-binding natural product with potent antiproliferative activity against several cancer cell lines that inhibits cell division by forming a covalent adduct with α-tubulin via a Michael addition into the natural product’s α,β-unsaturated lactone. We designed and prepared analogs carrying electron-withdrawing groups at the α-position (C2) of the α,β-unsaturated lactone with the goal to generate potent and selective binding analogs. We prepared derivatives containing halogens, a phenyl, and a methyl group at the C2 position to evaluate the structure-activity relationship at this position. Testing of the analogs in ovarian cancer cell lines demonstrated 100–1000-fold decreased antiproliferative activity.
中文翻译:

结合α-微管蛋白的天然产物吡罗宁的C2功能化类似物的合成和评价
吡咯丁酮是一种结合α-微管蛋白的天然产物,对几种癌细胞具有有效的抗增殖活性,通过将迈克尔·加成到自然产物的α,β-不饱和内酯中与α-微管蛋白形成共价加合物来抑制细胞分裂。我们设计并制备了在α,β-不饱和内酯的α-位置(C2)带有吸电子基团的类似物,目的是生成有效且选择性的结合类似物。我们制备了在C2位含有卤素,苯基和甲基的衍生物,以评估在此位置的构效关系。在卵巢癌细胞系中对类似物的测试表明其抗增殖活性降低了100-1000倍。









































 京公网安备 11010802027423号
京公网安备 11010802027423号