Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-11-10 , DOI: 10.1016/j.mcat.2017.11.001 Lina Zhang , Yingjie Hu , Mengwei Xue , Renming Pan , Bing Gao
|
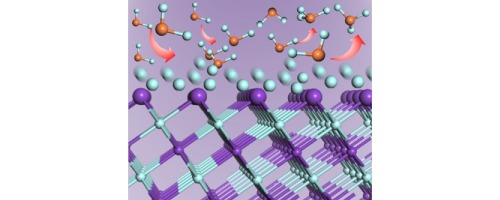
The surface chemistry of :CF2 over the KF(111) surface is systematically investigated using periodic, self-consistent, density functional theory (DFT), based on experimental results and elucidated catalysis reaction pathways as a “fluorine pool” mechanism. In agreement with experiment, with the help of density functional theory (DFT) calculations, we find that :CF2 and ⋮CF chemisorb on KF(111)-Kn surface is available and their C



中文翻译:

CF 2转化为KF(111)表面上CF 3反应途径的第一性原理和实验研究
:KF(111)表面上:CF 2的表面化学性质基于实验结果和阐明的催化反应途径(作为“氟库”机制),使用周期性,自洽,密度泛函理论(DFT)进行了系统研究。与实验相一致,借助密度泛函理论(DFT)计算,我们发现:在KF(111)-K n表面上可用:CF 2和⋮CF化学吸附,并且它们的C F键被fcc和hcp激活空洞。在C F活化下,:CF 2的两步脱氟反应容易产生氟和吸附在KF(111)-K n上的碳原子,从而导致氟原子覆盖KF(111)-K












































 京公网安备 11010802027423号
京公网安备 11010802027423号