Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-11-10 , DOI: 10.1016/j.mcat.2017.11.006 Getachew S. Molla , Alexander Himmelspach , Roland Wohlgemuth , Erhard T.K. Haupt , Andreas Liese
|
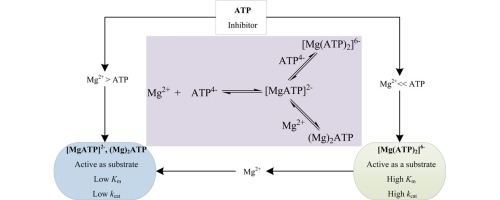
Detailed kinetics and mechanistic analyses of glycerol kinase from Cellulomonas sp. with respect to Mg2+ to ATP molar ratio were performed. The enzyme essentially requires Mg2+ for its activity and shows maximum activity at the optimum Mg2+ to ATP molar ratio of [0.12–0.3]. Subsequent increase of Mg2+ to ATP molar ratio higher than the values in the optimum region suppresses the enzyme activity to a non-zero asymptotic value. The enzyme exhibits two-step kinetics as a function of ATP at a fixed Mg2+ concentration due to the formation of multiple Mg-ATP complexes at different Mg2+ to ATP molar ratio. The addition of inorganic polyphosphate (PPin) inhibits or activates glycerol kinase due to the complexation of PPin with Mg2+ that shifts Mg2+ to ATP molar ratio below or to the optimum level. The change in all 31P NMR signals of ATP (i.e. α-, β- and γ-phosphate) by the addition of Mg2+ reveals that all of them are involved in Mg-ATP complex formation. The 1H NMR signals of CH2-protons on the ribose moiety of ATP become nearly equivalent after the addition of Mg2+ establishing the upper limit of optimum Mg2+ to ATP molar ratio for the enzyme activity. Therefore, it is concluded that the active site of glycerol kinase shows different catalytic property with respect to different Mg-ATP complexes. Glycerol kinase exhibits high affinity (low Km) and less activity (low kcat) for complexes with a stoichiometric or over-stoichiometric constitution like Mg2ATP. On the other hand, the enzyme shows less affinity (high Km) and high activity (high kcat) for unsaturated complexes like [Mg(ATP)2]−6.
中文翻译:

力学和动力学阐明Mg 2+ / ATP摩尔比对甘油激酶的影响
Cellulomonas sp。的甘油激酶的详细动力学和机理分析。关于Mg 2+与ATP的摩尔比进行。该酶基本上需要Mg 2+才能发挥其活性,并且在最佳Mg 2+与ATP摩尔比为[0.12-0.3]时显示出最大的活性。随后,Mg 2+与ATP摩尔比的增加高于最佳区域中的值,则将酶活性抑制为一个非零的渐近值。由于在不同的Mg 2+与ATP摩尔比下形成了多个Mg-ATP络合物,因此在固定的Mg 2+浓度下,该酶表现出两步动力学作为ATP的函数。无机多磷酸盐(PP in)由于PP i n与Mg 2+的络合而抑制或激活了甘油激酶,Mg 2+使Mg 2+与ATP的摩尔比降低至或低于最佳水平。通过添加Mg 2+改变ATP的所有31 P NMR信号(即α-,β-和γ-磷酸盐)的变化表明,它们全部都与Mg-ATP络合物的形成有关。加入Mg 2+确立了最佳Mg 2+的上限后,ATP核糖部分上CH 2质子的1 H NMR信号几乎相等。与ATP的摩尔比表示酶的活性。因此,可以得出结论,相对于不同的Mg-ATP复合物,甘油激酶的活性位点显示出不同的催化性能。甘油激酶对具有化学计量或化学计量过量组成的复合物(例如Mg 2 ATP )表现出高亲和力(低 K m)和较低活性(低k cat)。另一方面,该酶对[Mg(ATP)2 ] -6等不饱和复合物显示出较低的亲和力(较高的 K m)和较高的活性(较高的k cat)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号