Molecular Catalysis ( IF 4.6 ) Pub Date : 2017-11-10 , DOI: 10.1016/j.mcat.2017.10.021 A. Wirwis , J. Feder-Kubis , A.M. Trzeciak
|
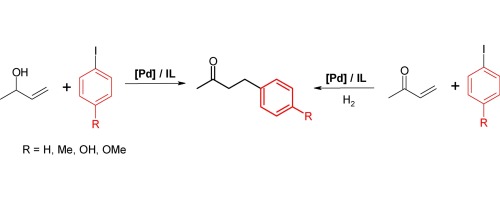
Allylic alcohols, 1-buten-3-ol, 1-penten-3-ol and 1-octen-3-ol, reacted with aryl iodides (iodotoluene, 4-iodotoluene, 4-iodophenol and 4-iodanisole) under Heck reaction conditions to form corresponding saturated aryl ketones in one step. The same products were obtained in a two-step tandem reaction consisted of the Heck coupling of allylic alcohols with aryl iodides, followed by hydrogenation. Reactions were catalyzed by phosphorus-free palladium precursors modified with the menthol-substituted imidazolium chlorides. Formation of crystalline palladium nanoparticles, of the diameter up to 65 nm, in the reaction mixture was evidenced by TEM.
中文翻译:

无磷钯催化剂催化合成芳基酮的两种有效途径
在Heck反应条件下,烯丙醇1-丁烯-3-醇,1-戊烯-3-醇和1-辛烯-3-醇与芳基碘化物(碘代甲苯,4-碘代甲苯,4-碘苯酚和4-碘苯甲醚)反应一步形成相应的饱和芳基酮 在两步串联反应中获得了相同的产物,该反应由烯丙基醇与芳基碘化物的Heck偶联组成,然后进行氢化。通过用薄荷醇取代的咪唑鎓氯化物改性的无磷钯前体催化反应。通过TEM证实在反应混合物中形成直径高达65nm的结晶钯纳米颗粒。



























 京公网安备 11010802027423号
京公网安备 11010802027423号