Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-10-06 , DOI: 10.1016/j.mcat.2017.09.028 Xiaoxia Han , Wei Sun , Chaofan Zhao , Ruina Shi , Xuhui Wang , Shusen Liu , Zhong Li , Jun Ren
|
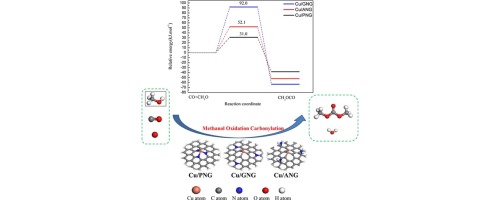
The synthesis of dimethyl carbonate (DMC) by oxidative carbonylation of methanol on atomic Cu supported on N-doped graphene (Cu1/NG) was explored by density-functional theory. The configurations with which the Cu atom is anchored to pyridinic- (Cu/PNG), graphitic- (Cu/GNG) and amino-N (Cu/ANG) doped graphene have been systematically investigated and compared with Cu-doped monovacancy graphene (Cu/MG) and pristine graphene (Cu/PG). The binding energy of the Cu atom was found to decrease in the order Cu/GNG > Cu/ANG > Cu/MG > Cu/PNG > Cu/PG. On Cu1/NG, DMC can be formed via CO insertion into CH3O to form CH3OCO, which then reacts with additional methoxide. The barrier energies of the rate-limiting reaction of CO insertion into methoxide on Cu/PNG, Cu/GNG, Cu/MG, and Cu/ANG surfaces have been found to be 31.0, 52.1, 73.5, and 92.0 kJ/mol, respectively. And the corresponding reaction energies are −38.2, −51.3, −44.6, and −63.4 kJ/mol, respectively. The activity of the Cu1/NG catalysts increases in the order Cu/PNG < Cu/GNG < Cu/MG < Cu/ANG. To summarize, the presence of pyridinic- and graphitic-N in Cu1/NG catalysts is beneficial to the oxidative carbonylation of methanol, while amino-N has a negative effect on DMC formation.
中文翻译:

N掺杂石墨烯中单Cu原子上碳酸二甲酯的合成:氮的影响
利用密度泛函理论研究了甲醇在氮掺杂石墨烯(Cu 1 / NG)上负载的Cu上的羰基氧化反应合成碳酸二甲酯(DMC)的方法。已经系统地研究了将Cu原子锚定于吡啶-(Cu / PNG),石墨-(Cu / GNG)和氨基N(Cu / ANG)掺杂石墨烯的构型,并将其与掺杂Cu的单空位石墨烯(Cu / MG)和原始石墨烯(Cu / PG)。发现Cu原子的结合能以Cu / GNG> Cu / ANG> Cu / MG> Cu / PNG> Cu / PG的顺序降低。对Cu 1 / NG,DMC可以经由CO插入形成为CH 3 O操作形成CH 3OCO,然后与其他甲醇反应。已发现在Cu / PNG,Cu / GNG,Cu / MG和Cu / ANG表面上CO插入甲醇中的限速反应的势垒能分别为31.0、52.1、73.5和92.0 kJ / mol。 。并且相应的反应能分别为-38.2,-51.3,-44.6和-63.4kJ / mol。Cu 1 / NG催化剂的活性按Cu / PNG <Cu / GNG <Cu / MG <Cu / ANG的顺序增加。总而言之,Cu 1 / NG催化剂中吡啶-和石墨-N的存在有利于甲醇的氧化羰基化,而氨基-N对DMC的形成具有负面影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号