Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-11-10 , DOI: 10.1016/j.mcat.2017.09.017 Eugeny V. Starokon , Sergei E. Malykhin , Mikhail V. Parfenov , Georgy M. Zhidomirov , Alexander S. Kharitonov
|
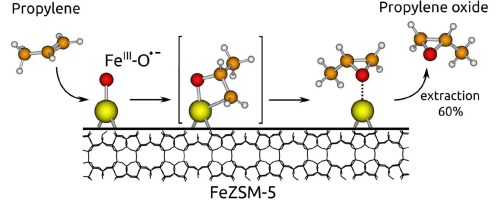
Reactions of anion-radical α-oxygen (FeIII–O•−)α with propylene and 1-butene on sodium-modified FeZSM-5 zeolites were studied in the temperature range from −60 to 25 °C. Products were extracted from the zeolite surface and identified. It was found that main reaction pathway was the epoxides formation. Selectivity for epoxides at −60 °C was 59–64%. Other products were formed as a result of secondary transformations of epoxides on the zeolite surface. According to IR spectroscopy, the oxidation of propylene over the entire temperature range and 1-butene at −60 °C were not accompanied by the formation of (FeIII–OH)α groups, in distinction to methane oxidation. This testifies that hydrogen abstraction does not occur. In case of 1-butene reaction with α-oxygen at 25 °C, hydrogen abstraction occurred but was insignificant, ca 7%. According to DFT calculation ferraoxetane intermediate formation is preferable over hydrogen abstraction. Following decomposition of this intermediate leads to the propylene oxide (PO) formation. The results may be relevant to the low selectivity problem of the silver catalyst in propylene epoxidation and raise doubts about the presently accepted mechanism explaining an adverse effect of allylic hydrogen.
中文翻译:

FeZSM -5表面上的α-氧(Fe III –O •−)α对低级烯烃的氧化:是环氧化还是烯丙基氧化?
在-60至25°C的温度范围内,研究了阴离子自由基α-氧(Fe III –O •−)α与丙烯和1-丁烯在钠改性的FeZSM-5沸石上的反应。从沸石表面提取产物并鉴定。发现主要的反应途径是环氧化物的形成。在−60°C下对环氧化物的选择性为59–64%。沸石表面上环氧化物二次转化的结果是形成了其他产物。根据红外光谱,在整个温度范围内,丙烯氧化和在-60°C下的1-丁烯氧化都不会伴随着(Fe III -OH)α的形成。基团,区别于甲烷氧化。这证明没有发生氢提取。在25°C下1-丁烯与α-氧反应的情况下,发生了夺氢,但微不足道,约为7%。根据DFT计算,铁氧杂环丁烷中间体的形成优于氢的夺取。该中间体的分解之后导致环氧丙烷(PO)的形成。该结果可能与丙烯环氧化中银催化剂的低选择性问题有关,并引起人们对目前公认的解释烯丙基氢的不利作用的机理的怀疑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号