Catalysis Today ( IF 5.2 ) Pub Date : 2017-11-11 , DOI: 10.1016/j.cattod.2017.11.008 Keita Taniya , Chih Hao Yu , Hiromu Takado , Taiki Hara , Atsushi Okemoto , Takafumi Horie , Yuichi Ichihashi , Shik Chi Tsang , Satoru Nishiyama
|
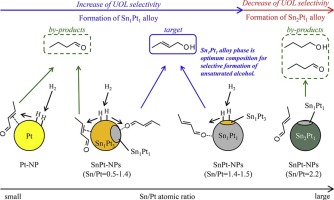
Bimetallic SnPt-nanoparticle (SnPt-NP) catalysts with several types of SnxPty alloy structures were prepared using a polyalcohol reduction process; the catalytic behavior of each SnxPty alloy phase toward the selective hydrogenation of unsaturated aldehydes to the corresponding unsaturated alcohols was elucidated. Atomic absorption spectroscopy (AAS) and transmission electron microscopy (TEM) indicate that SnPt-NP catalysts with various Sn/Pt atomic ratios can be successfully prepared by a polyalcohol reduction process using Pt(acac)2 and Sn(AcO)2 as the metal precursors. X-ray diffraction (XRD) results reveal that the Sn1Pt3, Sn1Pt1, and Sn2Pt1 alloy phases are formed through control of the Sn/Pt atomic ratio of the starting mixture during preparation. The Sn1Pt3 alloy phase enhanced the hydrogenation of both the CC and C
O bonds during the selective hydrogenation of crotonaldehyde. When only Sn1Pt1 alloy phases (Sn/Pt = 1.40), accompanying with separated Sn phase, were formed in the SnPt-NP catalysts, the highest unsaturated alcohol (UOL) selectivity (71.5% at 37.6% conversion) was observed. The formation of the Sn2Pt1 alloy phase led to decreased UOL selectivity. We suggest that the Sn1Pt1 alloy phase is an effective bimetallic SnPt hydrogenation catalyst for the selective formation of unsaturated alcohols.
中文翻译:

巴豆醛化学选择加氢的双金属SnPt-纳米颗粒催化剂的合成:Sn x Pt y合金相与催化性能之间的关系
采用多元醇还原法制备了具有几种类型的Sn x Pt y合金结构的双金属SnPt-纳米颗粒(SnPt-NP)催化剂。阐明了每个Sn x Pt y合金相对不饱和醛选择性加氢为相应的不饱和醇的催化行为。原子吸收光谱法(AAS)和透射电子显微镜(TEM)表明,采用Pt(acac)2和Sn(AcO)2作为金属的多元醇还原工艺可以成功制备具有不同Sn / Pt原子比的SnPt-NP催化剂。前体。X射线衍射(XRD)结果表明,Sn 1 Pt 3,Sn通过控制制备过程中起始混合物的Sn / Pt原子比,形成1 Pt 1和Sn 2 Pt 1合金相。Sn 1 Pt 3合金相在巴豆醛的选择性加氢过程中增强了C C和C
O键的加氢作用。当在SnPt-NP催化剂中仅形成Sn 1 Pt 1合金相(Sn / Pt = 1.40)并伴有分离的Sn相时,观察到最高的不饱和醇(UOL)选择性(在37.6%的转化率下为71.5%)。Sn 2 Pt 1合金相的形成导致UOL选择性降低。我们建议锡1 Pt 1合金相是一种有效的双金属SnPt加氢催化剂,用于选择性形成不饱和醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号