当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for the Serratia marcescens Lipase Secretion System: Crystal Structures of the Membrane Fusion Protein and Nucleotide-Binding Domain
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.biochem.7b00985 Daichi Murata 1 , Hiroyuki Okano 2 , Clement Angkawidjaja 2 , Masato Akutsu 3 , Shun-ichi Tanaka 4 , Kenyu Kitahara 1 , Takuya Yoshizawa 4 , Hiroyoshi Matsumura 4 , Yuji Kado 2 , Eiichi Mizohata 2 , Tsuyoshi Inoue 2 , Satoshi Sano 1 , Yuichi Koga 2 , Shigenori Kanaya 2 , Kazufumi Takano 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.biochem.7b00985 Daichi Murata 1 , Hiroyuki Okano 2 , Clement Angkawidjaja 2 , Masato Akutsu 3 , Shun-ichi Tanaka 4 , Kenyu Kitahara 1 , Takuya Yoshizawa 4 , Hiroyoshi Matsumura 4 , Yuji Kado 2 , Eiichi Mizohata 2 , Tsuyoshi Inoue 2 , Satoshi Sano 1 , Yuichi Koga 2 , Shigenori Kanaya 2 , Kazufumi Takano 1
Affiliation
|
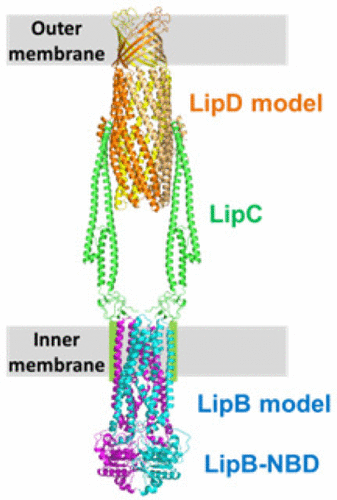
Serratia marcescens secretes a lipase, LipA, through a type I secretion system (T1SS). The T1SS for LipA, the Lip system, is composed of an inner membrane ABC transporter with its nucleotide-binding domains (NBD), LipB, a membrane fusion protein, LipC, and an outer membrane channel protein, LipD. Passenger protein secreted by this system has been functionally and structurally characterized well, but relatively little information about the transporter complex is available. Here, we report the crystallographic studies of LipC without the membrane anchor region, LipC-, and the NBD of LipB (LipB-NBD). LipC- crystallographic analysis has led to the determination of the structure of the long α-helical and lipoyl domains, but not the area where it interacts with LipB, suggesting that the region is flexible without LipB. The long α-helical domain has three α-helices, which interacts with LipD in the periplasm. LipB-NBD has the common overall architecture and ATP hydrolysis activity of ABC transporter NBDs. Using the predicted models of full-length LipB and LipD, the overall structural insight into the Lip system is discussed.
中文翻译:

粘质沙雷氏菌脂肪酶分泌系统的结构基础:膜融合蛋白和核苷酸结合域的晶体结构。
粘质沙雷氏菌通过I型分泌系统(T1SS)分泌脂肪酶LipA。LipA的T1SS,即Lip系统,由内膜ABC转运蛋白及其核苷酸结合结构域(NBD)LipB,膜融合蛋白LipC和外膜通道蛋白LipD组成。该系统分泌的客运蛋白已经在功能和结构上得到了很好的表征,但是关于转运蛋白复合物的信息相对较少。在这里,我们报告没有膜锚定区域,LipC-和LipB的NBD(LipB-NBD)的LipC的晶体学研究。LipC晶体学分析已确定了长α-螺旋和脂酰结构域的结构,但未确定其与LipB相互作用的区域,这表明该区域在没有LipB的情况下是柔性的。长的α螺旋域具有三个α螺旋,它与周质中的LipD相互作用。LipB-NBD具有ABC转运蛋白NBD的通用总体结构和ATP水解活性。使用全长LipB和LipD的预测模型,讨论了对Lip系统的整体结构见解。
更新日期:2017-11-11
中文翻译:

粘质沙雷氏菌脂肪酶分泌系统的结构基础:膜融合蛋白和核苷酸结合域的晶体结构。
粘质沙雷氏菌通过I型分泌系统(T1SS)分泌脂肪酶LipA。LipA的T1SS,即Lip系统,由内膜ABC转运蛋白及其核苷酸结合结构域(NBD)LipB,膜融合蛋白LipC和外膜通道蛋白LipD组成。该系统分泌的客运蛋白已经在功能和结构上得到了很好的表征,但是关于转运蛋白复合物的信息相对较少。在这里,我们报告没有膜锚定区域,LipC-和LipB的NBD(LipB-NBD)的LipC的晶体学研究。LipC晶体学分析已确定了长α-螺旋和脂酰结构域的结构,但未确定其与LipB相互作用的区域,这表明该区域在没有LipB的情况下是柔性的。长的α螺旋域具有三个α螺旋,它与周质中的LipD相互作用。LipB-NBD具有ABC转运蛋白NBD的通用总体结构和ATP水解活性。使用全长LipB和LipD的预测模型,讨论了对Lip系统的整体结构见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号