Joule ( IF 39.8 ) Pub Date : 2017-10-16 , DOI: 10.1016/j.joule.2017.09.014 Xueli Zheng , Phil De Luna , F. Pelayo García de Arquer , Bo Zhang , Nigel Becknell , Michael B. Ross , Yifan Li , Mohammad Norouzi Banis , Yuzhang Li , Min Liu , Oleksandr Voznyy , Cao Thang Dinh , Taotao Zhuang , Philipp Stadler , Yi Cui , Xiwen Du , Peidong Yang , Edward H. Sargent
|
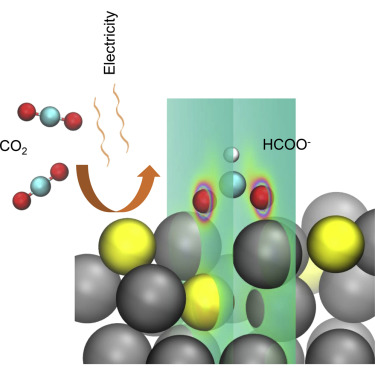
Electrochemical reduction of carbon dioxide (CO2RR) to formate provides an avenue to the synthesis of value-added carbon-based fuels and feedstocks powered using renewable electricity. Here, we hypothesized that the presence of sulfur atoms in the catalyst surface could promote undercoordinated sites, and thereby improve the electrochemical reduction of CO2 to formate. We explored, using density functional theory, how the incorporation of sulfur into tin may favor formate generation. We used atomic layer deposition of SnSx followed by a reduction process to synthesize sulfur-modulated tin (Sn(S)) catalysts. X-ray absorption near-edge structure (XANES) studies reveal higher oxidation states in Sn(S) compared with that of tin in Sn nanoparticles. Sn(S)/Au accelerates CO2RR at geometric current densities of 55 mA cm−2 at −0.75 V versus reversible hydrogen electrode with a Faradaic efficiency of 93%. Furthermore, Sn(S) catalysts show excellent stability without deactivation (<2% productivity change) following more than 40 hours of operation.
中文翻译:

硫调节锡位点可实现高选择性的电化学还原,将CO 2还原为甲酸酯
电化学还原二氧化碳(CO 2 RR)生成甲酸盐为合成利用可再生电力驱动的增值碳基燃料和原料提供了一条途径。在这里,我们假设催化剂表面硫原子的存在可以促进配位不足,从而改善CO 2的电化学还原生成甲酸酯。我们使用密度泛函理论探讨了将硫掺入锡中如何促进甲酸盐的生成。我们使用了原子层沉积的SnS x然后进行还原过程以合成硫调节的锡(Sn(S))催化剂。X射线吸收近边缘结构(XANES)研究表明,与锡纳米颗粒中的锡相比,锡(S)中的氧化态更高。与可逆氢电极相比,Sn(S)/ Au在-0.75 V的几何电流密度为55 mA cm -2时可加速CO 2 RR,法拉第效率为93%。此外,Sn(S)催化剂在运行40多个小时后仍表现出出色的稳定性,而不会失活(<2%的生产率变化)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号