Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.mcat.2017.09.016 F.J. Delgado-Gómez , V. Calvino-Casilda , A. Cerpa-Naranjo , M.L. Rojas-Cervantes
|
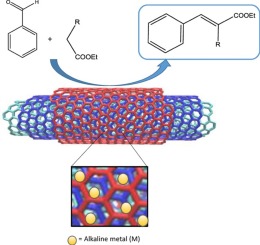
A series of alkaline doped multiwall carbon nanotubes, CNTOM (with M = Li, Na, K, Cs) has been prepared by wetness impregnation with alkaline acetates and subsequent pyrolysis of commercial multiwall carbon nanotubes previously oxidized with nitric acid, characterized by different physico-chemical techniques and tested as catalysts in the Knoevenagel condensation between benzaldehyde and different active methylene compounds. As determined by different techniques, caesium has been incorporated in a less extent than the rest of alkalines. The SBET values decrease in the CNTOM with respect to the raw material, mainly due to the blockage of micropores and small mesopores by the metallic phase formed. As deduced from PZC values, the character of the surface of nanotubes changes from acid for the oxidized sample to basic for the alkaline doped nanotubes, the basic character decreasing with the size of the alkaline cation. The order of activity observed in the Knoevenagel condensation between benzaldehyde and ethyl cyanoacetate seems to be correlated mainly with the amount of basic centres, CNTOCs being the less active catalyst due to its lower metal content. Conversion values of benzaldehyde comprised between 67 and 89% were achieved in only 120 min.
中文翻译:

碱性掺杂多壁碳纳米管可作为Knoevenagel缩合反应的有效催化剂
一系列碱性掺杂的多壁碳纳米管CNTOM(M = Li,Na,K,Cs)的制备是通过用碱性乙酸酯进行湿法浸渍,然后将先前用硝酸氧化的商业化多壁碳纳米管进行热解,其特征在于不同的物理化学性质。化学技术,并在苯甲醛与不同活性亚甲基化合物之间的Knoevenagel缩合反应中作为催化剂进行测试。如通过不同的技术所确定的,铯的掺入量比其余碱性物质的掺入量要少。在S BET相对于原料,CNTOM值降低,主要是由于形成的金属相阻塞了微孔和小中孔。从PZC值推导,纳米管表面的特性从被氧化样品的酸变为碱性掺杂纳米管的碱性,该碱性特性随碱性阳离子的大小而降低。在苯甲醛和氰基乙酸乙酯之间的Knoevenagel缩合反应中观察到的活性顺序似乎主要与碱性中心的数量有关,CNTOCs由于金属含量较低而成为活性较低的催化剂。仅120分钟就达到了介于67%和89%之间的苯甲醛的转化率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号