Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.mcat.2017.10.004 Jia-Qi Liu , Jun-Juan Yang , Jun-Fei Li , Kai Li , Xue-Dong Xiao , Ya-Li Bai , Jun-Wen Wang
|
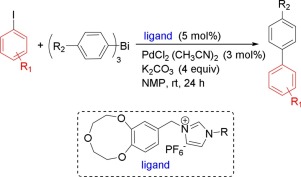
We have developed the N-heterocyclic carbene ligand/PdCl2 catalyst for CC coupling reaction of aryl iodides and organobismuths at room temperature. The established catalytic system exhibited high cross-coupling reactivity between a variety of orgnobismuths and aryl iodides in the presence of K2CO3 as base in NMP or DMSO at room temperature. The simple and efficient transformation can tolerate either electron-withdrawing or electron-donating functional groups. It was notably found that both aryl bromide and aryl chloride generated moderate to good yields of the corresponding biphenyl products using 5 mol% of PPh3/ligand 5 (1:1) as catalyst.
中文翻译:

N-杂环卡宾-PdCl 2在苯并-9-冠-3催化剂上在室温下催化芳基碘化物与三芳基铋的交叉偶联反应
我们已经开发出用于在室温下芳基碘化物和有机铋的C C偶联反应的N杂环卡宾配体/ PdCl 2催化剂。所建立的催化体系在室温下在NMP或DMSO中存在以K 2 CO 3为碱的情况下,在各种正铋和芳基碘之间表现出较高的交叉偶联反应性。简单而有效的转化可以耐受吸电子或给电子官能团。值得注意的是,使用5mol%的PPh 3 /配体5(1∶1)作为催化剂,芳基溴化物和芳基氯均产生中等至良好产率的相应联苯产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号