Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.mcat.2017.10.023 Sunatda Arayachukiat , Prapussorn Yingcharoen , Sai V.C. Vummaleti , Luigi Cavallo , Albert Poater , Valerio D’Elia
|
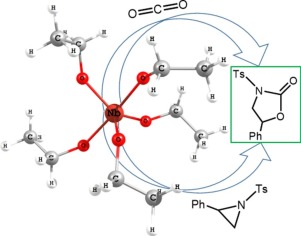
An efficient and facile approach to the regioselective synthesis of N-tosyloxazolidinones from the corresponding N-tosylaziridines and CO2 was developed using dual catalytic systems involving an early transition metal coordination compound as a Lewis acid and a nucleophilic cocatalyst. Among the screened Lewis acids, halogen-free niobium pentaethoxide (Nb(OEt)5) displayed the best catalytic activity when used in the presence of tetrabutylammonium iodide (TBAI). Systematic DFT calculations, supported by catalytic experiments, demonstrate that CO2 insertion is the rate determining step for this process and it is highly dependent on the steric hindrance at the niobium center.
中文翻译:

使用无卤铌络合物将CO 2环化成具有挑战性的N-甲苯磺酰基氮丙啶:催化活性和机理研究
利用双催化体系开发了一种有效且简便的方法,该方法可从相应的N-甲苯磺酰氮丙啶和CO 2进行区域选择性合成N-甲苯磺恶唑烷酮类化合物,该体系包括早期过渡金属配位化合物(路易斯酸)和亲核助催化剂。在经筛选的路易斯酸中,当在碘化四丁基铵(TBAI)的存在下使用时,无卤素的五乙醇化铌(Nb(OEt)5)表现出最佳的催化活性。由催化实验支持的系统DFT计算表明,CO 2的插入是该过程的速率决定步骤,并且高度依赖于铌中心的位阻。











































 京公网安备 11010802027423号
京公网安备 11010802027423号