Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.jcat.2017.09.009 Dan Wang , Yingnan Sun , Qingkun Shang , Xinyue Wang , Tongtong Guo , Hongyu Guan , Qin Lu
|
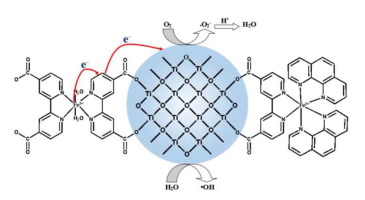
[FeII(dcbpy)2(H2O)2] (dcbpy = 2,2′-bipyridine-4,4′-dicarboxylic acid) complex was synthesized through a simple method and used as a photosensitizer to improve the photocatalytic activity of TiO2 in the photodegradation of phenol under visible light irradiation. X-ray diffraction, X-ray photoelectron spectroscopy, UV–vis spectroscopy, Raman spectra, scanning electron microscopy, and transmission electron microscopy were used to characterize the structure and morphology of [FeII(dcbpy)2(H2O)2]/TiO2. It exhibited enhanced photocatalytic activity in photodecomposition of phenol in aqueous solution under visible light irradiation compared with that of pure TiO2. When one of ligand dcbpy was replaced by bpy (2,2′-bipyridine) or phen (1,10-phenanthroline), two novel composite catalysts, [FeII(dcbpy)(bpy)2]/TiO2 and [FeII(dcbpy)(phen)2]/TiO2, were obtained and exhibited excellent photocatalytic activity like that of [FeII(dcbpy)2(H2O)2]/TiO2. Their different performance in the degradation of phenol, derived from their different interfacial interaction and electron transfer between complexes and TiO2 surface, are discussed in detail. Based on research on the dynamical process of photocatalytic degradation of phenol, recycling stability, absorption spectra, photoelectrochemistry performance and cyclic voltammetry, trapping experiments, and density functional theory calculations of quantum chemistry, possible electron transfer mechanism are proposed, which can reasonably explain the different photocatalytic activities of these three novel catalysts.
中文翻译:

Fe-联吡啶配合物的共轭结构对TiO 2光催化体系中光诱导电子转移的影响
[Fe II(dcbpy)2(H 2 O)2 ](dcbpy = 2,2'-联吡啶-4,4'-二羧酸)配合物是通过简单的方法合成的,并用作光敏剂以提高其的光催化活性。 TiO 2在可见光照射下光降解苯酚。X射线衍射,X射线光电子能谱,紫外可见光谱,拉曼光谱,扫描电子显微镜和透射电子显微镜被用来表征[Fe II(dcbpy)2(H 2 O)2 ]的结构和形态。/的TiO 2。与纯TiO 2相比,它在可见光照射下对水溶液中苯酚的光分解表现出增强的光催化活性。当配体dcbpy之一被bpy(2,2'-联吡啶)或phen(1,10-菲咯啉)取代时,两种新型复合催化剂[Fe II(dcbpy)(bpy)2 ] / TiO 2和[Fe II获得了(dcbpy)(phen)2 ] / TiO 2,并表现出优异的光催化活性,类似于[Fe II(dcbpy)2(H 2 O)2 ] / TiO 2。详细讨论了它们在界面降解和配合物与TiO 2表面之间的电子转移之间的不同相互作用,以及它们在苯酚降解方面的不同性能。在研究苯酚的光催化降解动力学过程,循环稳定性,吸收光谱,光电化学性能和循环伏安法,俘获实验以及量子化学的密度泛函理论计算的基础上,提出了可能的电子转移机理,可以合理地解释其不同之处。这三种新型催化剂的光催化活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号