Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.jcat.2017.09.011 Irina D. Ivanchikova , Igor Y. Skobelev , Nataliya V. Maksimchuk , Eugenii A. Paukshtis , Mikhail V. Shashkov , Oxana A. Kholdeeva
|
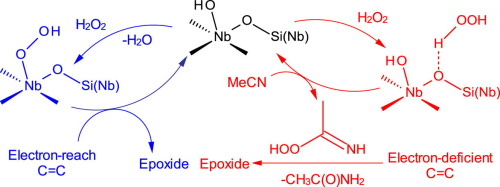
Niobium-containing mesoporous silicates reveal superior activity and selectivity in epoxidation of alkenes using hydrogen peroxide as a green oxidant and, in contrast to mesoporous titanium silicates, catalyze epoxidation of both electron-rich and electron-deficient CC bonds. This report describes a kinetic and mechanistic investigation of epoxidation of two representative substrates, cyclooctene (CyO) and 2-methyl-1,4-naphthoquinone (MNQ), over two mesoporous niobium silicates with predominantly di(oligo)meric or isolated Nb(V) sites. The observed kinetic regularities did not depend on the state of Nb but were strictly determined by the nature of the organic substrate. The rate law established for CyO is consistent with a mechanism that involves interaction of H2O2 with Nb(V) sites to give a hydroperoxo species NbOOH and water, followed by oxygen transfer to a nucleophilic C
C bond, producing an epoxide and regenerating the initial state of the catalyst. This mechanism is strongly supported by stereospecificity in epoxidation of cis-alkenes and high heterolytic pathway selectivity in the oxidation of cyclohexene. The NbOOH species is manifested by an absorption feature at 307 nm in diffuse reflectance UV–vis spectra. The addition of a base (NaOAc) causes a shift of the absorption band to 293 nm and produces a rate-retarding effect on the epoxidation reaction. Several lines of evidence, including zero reaction order in MNQ, rate-accelerating effect of base, detection of acetamide in the reaction mixture, negligible reaction rate in ethyl acetate, and recognition of weak basic sites in the niobium silicates using infrared spectroscopy of adsorbed CDCl3, all indicate that MNQ epoxidation proceeds by another mechanism that involves rate-limiting oxidation of the solvent molecule (MeCN) to generate peroxycarboximidic acid, which reacts with electron-deficient C
C bonds, producing an epoxy derivative and acetamide.
中文翻译:

理解介孔硅酸铌硅在C
含铌的介孔硅酸盐在使用过氧化氢作为绿色氧化剂的烯烃的环氧化中显示出优异的活性和选择性,并且与介孔硅酸钛相反,它催化富电子和缺电子的C C键的环氧化。该报告描述了在两种介孔硅酸铌硅酸盐上主要存在二(低聚)或分离的Nb(V)的两种代表性底物环辛烯(CyO)和2-甲基-1,4-萘醌(MNQ)的环氧化动力学和机理)网站。观察到的动力学规律不取决于Nb的状态,而是由有机底物的性质严格确定的。为CyO建立的速率定律与涉及H 2 O 2相互作用的机制是一致的用Nb(V)位点得到氢过氧物质NbOOH和水,然后将氧转移到亲核C
C键,产生环氧化物并再生催化剂的初始状态。该机制在顺式环氧化中的立体特异性得到了强有力的支持。-烯烃和环己烯氧化中的高杂化途径选择性。NbOOH种类在漫反射紫外可见光谱中在307 nm处具有吸收特征。碱(NaOAc)的添加会导致吸收带移至293 nm,并对环氧化反应产生速率降低的作用。多种证据,包括MNQ中的反应顺序为零,碱的速率加速作用,反应混合物中乙酰胺的检测,乙酸乙酯中可忽略的反应速率以及使用吸附的CDCl的红外光谱识别硅酸铌中的弱碱性位点3所有这些都表明,MNQ环氧化是通过另一种机理进行的,该机理涉及溶剂分子(MeCN)的限速氧化反应,以生成过氧羧酰亚胺酸,该过氧羧酰亚胺酸与缺电子的C
C键反应,生成环氧衍生物和乙酰胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号