Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-11-06 , DOI: 10.1016/j.jcat.2017.10.009 Yu Lou , Matthias Steib , Qi Zhang , Konrad Tiefenbacher , Anita Horváth , Andreas Jentys , Yue Liu , Johannes A. Lercher
|
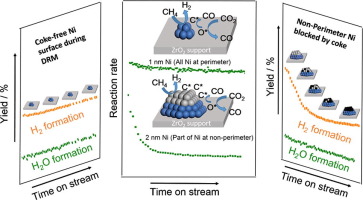
ZrO2 supported Ni with an average particle diameter of 1–2 nm were synthesized by binding Ni2+ in rccc-5,11,17,23-tetrahydroxy-2,8,14,20-tetra(n-decyl)resorcin[4]arene (pyrogallol[4]arene) nanocapsules as Ni precursor. The Ni/ZrO2 catalyst with a 1.1 nm particle diameter showed outstanding stability in dry reforming of methane (DRM), which maintained nearly 90% of the initial activity after 60 h time on stream. The high stability is attributed to nearly all Ni atoms being located at the interface and perimeter to ZrO2. This led to a higher accessibility to the oxygen from activated CO2 at the Ni-ZrO2 interface, facilitating conversion of surface carbon to CO. Ni/ZrO2 catalysts with larger Ni particle diameter have a fraction of non-perimeter Ni that deactivates rapidly. The decrease of the H2 formation rate was faster with time on stream than the decrease of the H2O formation rate. At longer time on stream the ratio of H2O to H2 yield reached 0.40 ± 0.08 for all Ni/ZrO2 catalysts, independently if a catalyst was stable from the reaction start or deactivated to a stable level. The ratio between H2O and H2 yield reflects the abundance of oxygen availability on the Ni surface, the oxygen availability index (OAI). For an OAI value of 0.40, the deactivation of Ni catalyst was negligible, while below that deactivation was pronounced. The reorganization of surface carbon to graphitic overlayers and carbon fibers is hypothesized to start from surface domains that are not adjacent to the metal-support perimeter.
中文翻译:

用于甲烷干重整的稳定Ni / ZrO 2催化剂的设计
通过将Ni 2+结合在rccc-5,11,17,23-四羟基-2,8,14,20-四(正癸基)间苯二酚中,合成了ZrO 2负载的Ni,其平均粒径为1-2 nm。4]芳烃(间苯三酚[4]芳烃)纳米胶囊为镍前驱体。粒径为1.1 nm的Ni / ZrO 2催化剂在甲烷干重整(DRM)中表现出出色的稳定性,在运行60小时后,其可保持近90%的初始活性。高稳定性归因于几乎所有的Ni原子都位于ZrO 2的界面和周边。这导致从Ni-ZrO 2界面处的活化CO 2接触氧气的可能性更高,从而促进了表面碳向CO的转化。Ni / ZrO 2Ni粒径较大的催化剂的非周边Ni分数会迅速失活。H 2形成速率的降低随运行时间的增加比H 2 O形成速率的降低要快。对于所有Ni / ZrO 2催化剂,在较长的运行时间中,H 2 O与H 2的收率之比达到0.40±0.08 ,如果催化剂从反应开始就稳定或失活到稳定水平,则与之无关。H 2 O与H 2的比例产量反映了镍表面上充足的氧气可利用性,即氧气可利用性指数(OAI)。对于OAI值为0.40,Ni催化剂的失活可忽略不计,而低于该值则显着失活。假设将表面碳重组为石墨覆盖层和碳纤维是从与金属载体周边不相邻的表面区域开始的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号