The Lancet Psychiatry ( IF 30.8 ) Pub Date : 2017-10-04 , DOI: 10.1016/s2215-0366(17)30371-1 Paul E Holtzheimer , Mustafa M Husain , Sarah H Lisanby , Stephan F Taylor , Louis A Whitworth , Shawn McClintock , Konstantin V Slavin , Joshua Berman , Guy M McKhann , Parag G Patil , Barry R Rittberg , Aviva Abosch , Ananda K Pandurangi , Kathryn L Holloway , Raymond W Lam , Christopher R Honey , Joseph S Neimat , Jaimie M Henderson , Charles DeBattista , Anthony J Rothschild , Julie G Pilitsis , Randall T Espinoza , Georgios Petrides , Alon Y Mogilner , Keith Matthews , DeLea Peichel , Robert E Gross , Clement Hamani , Andres M Lozano , Helen S Mayberg
|
Background
Deep brain stimulation (DBS) of the subcallosal cingulate white matter has shown promise as an intervention for patients with chronic, unremitting depression. To test the safety and efficacy of DBS for treatment-resistant depression, a prospective, randomised, sham-controlled trial was conducted.
Methods
Participants with treatment-resistant depression were implanted with a DBS system targeting bilateral subcallosal cingulate white matter and randomised to 6 months of active or sham DBS, followed by 6 months of open-label subcallosal cingulate DBS. Randomisation was computer generated with a block size of three at each site before the site started the study. The primary outcome was frequency of response (defined as a 40% or greater reduction in depression severity from baseline) averaged over months 4–6 of the double-blind phase. A futility analysis was performed when approximately half of the proposed sample received DBS implantation and completed the double-blind phase. At the conclusion of the 12-month study, a subset of patients were followed up for up to 24 months. The study is registered at ClinicalTrials.gov, number NCT00617162.
Findings
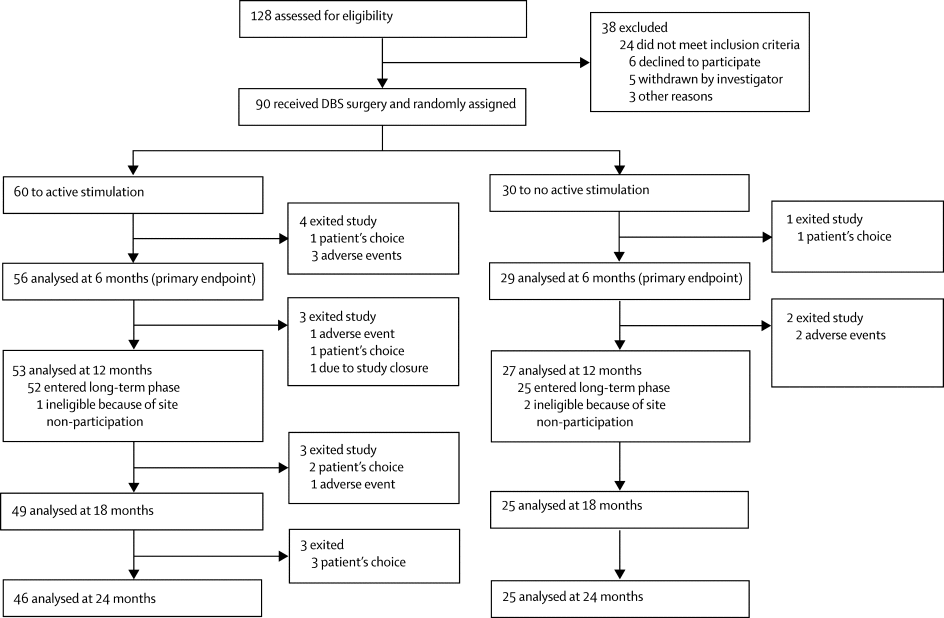
Before the futility analysis, 90 participants were randomly assigned to active (n=60) or sham (n=30) stimulation between April 10, 2008, and Nov 21, 2012. Both groups showed improvement, but there was no statistically significant difference in response during the double-blind, sham-controlled phase (12 [20%] patients in the stimulation group vs five [17%] patients in the control group). 28 patients experienced 40 serious adverse events; eight of these (in seven patients) were deemed to be related to the study device or surgery.
Interpretation
This study confirmed the safety and feasibility of subcallosal cingulate DBS as a treatment for treatment-resistant depression but did not show statistically significant antidepressant efficacy in a 6-month double-blind, sham-controlled trial. Future studies are needed to investigate factors such as clinical features or electrode placement that might improve efficacy.
Funding
Abbott (previously St Jude Medical).
中文翻译:

call突肌下扣带回深脑刺激治疗难治性抑郁症:一项多站点,随机,假对照试验
背景
call愈后扣带回白质的深层脑刺激(DBS)已显示出有望作为对慢性,持续性抑郁症患者的一种干预措施。为了测试DBS治疗难治性抑郁症的安全性和有效性,进行了一项前瞻性,随机,假对照试验。
方法
患有难治性抑郁症的参与者被植入针对双侧call骨扣带回扣状白质的DBS系统,并随机分配至6个月的活动性或假性DBS,然后进行6个月的开放标签sub骨扣带回扣状DBS。随机化是由计算机生成的,在该站点开始研究之前,每个站点的块大小为3。主要结果是在双盲阶段的第4到6个月内,平均的响应频率(定义为抑郁症的严重程度较基线水平降低40%或更多)。当大约一半的拟议样品接受DBS植入并完成双盲阶段时,进行了无效分析。在为期12个月的研究结束时,对部分患者进行了长达24个月的随访。该研究已在ClinicalTrials.gov上注册,编号为NCT00617162。
发现
在无效性分析之前,在2008年4月10日至2012年11月21日之间,将90名参与者随机分配为主动(n = 60)或假(n = 30)刺激。两组均表现出改善,但在统计学上无统计学差异。在双盲假手术控制阶段的反应(刺激组为12 [20%]患者,对照组为5 [17%])。28例患者发生了40例严重不良事件;其中八名患者(七名患者)被认为与研究设备或手术有关。
解释
这项研究证实了call骨扣带回DBS作为抗药性抑郁症的治疗方法的安全性和可行性,但在为期6个月的双盲,假对照试验中,未显示出统计学上显着的抗抑郁功效。需要进行进一步的研究来调查可能会提高疗效的因素,例如临床特征或电极放置。
资金
雅培(以前是St Jude Medical)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号