当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective palladium-catalyzed C–H functionalization of pyrroles using an axially chiral 2,2′-bipyridine ligand
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7qo00815e Hong-Qiang Shen 1, 2, 3, 4, 5 , Cong Liu 1, 2, 3, 4, 5 , Ji Zhou 1, 2, 3, 4, 5 , Yong-Gui Zhou 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7qo00815e Hong-Qiang Shen 1, 2, 3, 4, 5 , Cong Liu 1, 2, 3, 4, 5 , Ji Zhou 1, 2, 3, 4, 5 , Yong-Gui Zhou 1, 2, 3, 4, 5
Affiliation
|
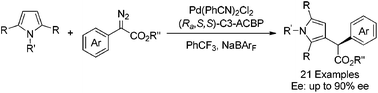
An enantioselective C–H functionalization of pyrrole derivatives with diazo compounds has been successfully realized with up to 90% ee by employing dichlorobis(benzonitrile)palladium with an axially chiral bipyridine ligand C3-ACBP as the catalyst. Asymmetric C–H functionalization at the gram scale was also conducted smoothly with good reactivity and enantioselectivity.
中文翻译:

使用轴向手性2,2'-联吡啶配体的对映体钯催化吡咯的CH-H官能化
通过使用二氯双(苄腈)钯和轴向手性联吡啶配体C3-ACBP作为催化剂,成功地实现了重氮对吡咯衍生物与重氮化合物的对映选择性C–H功能化,收率高达90%。克级的不对称C–H功能化也能顺利进行,并具有良好的反应性和对映选择性。
更新日期:2017-11-10
中文翻译:

使用轴向手性2,2'-联吡啶配体的对映体钯催化吡咯的CH-H官能化
通过使用二氯双(苄腈)钯和轴向手性联吡啶配体C3-ACBP作为催化剂,成功地实现了重氮对吡咯衍生物与重氮化合物的对映选择性C–H功能化,收率高达90%。克级的不对称C–H功能化也能顺利进行,并具有良好的反应性和对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号