当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Specificity of Donor Structures for endo‐β‐N‐Acetylglucosaminidase‐Catalyzed Transglycosylation Reactions
ChemBioChem ( IF 3.2 ) Pub Date : 2017-12-04 , DOI: 10.1002/cbic.201700506 Nozomi Ishii 1 , Ken Ogiwara 1 , Kanae Sano 1 , Jyunichi Kumada 2 , Kenji Yamamoto 3 , Yuji Matsuzaki 2 , Ichiro Matsuo 1
ChemBioChem ( IF 3.2 ) Pub Date : 2017-12-04 , DOI: 10.1002/cbic.201700506 Nozomi Ishii 1 , Ken Ogiwara 1 , Kanae Sano 1 , Jyunichi Kumada 2 , Kenji Yamamoto 3 , Yuji Matsuzaki 2 , Ichiro Matsuo 1
Affiliation
|
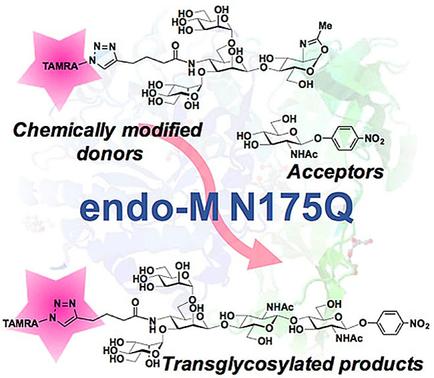
The sweet spot: The structural specificity of the glycosyl donor for the transglycosylation of endo‐β‐N‐acetylglucosaminidase from M. hiemalis (endo‐M) and its mutants, N175Q and N175A, is investigated by using unnatural tetrasaccharide derivatives. Transglycosylation with donors chemically modified at the C‐4 position of the β‐mannose residue is examined, and transglycosylated products are successfully obtained.

中文翻译:

供体β-N-乙酰氨基葡萄糖氨基糖苷酶催化的转糖基化反应结构的特异性
关键点:使用非天然四糖衍生物研究了糖基施主对hi.alis (hiemalis)(endo- M)的内-β- N-乙酰氨基葡糖苷酶及其突变体N175Q和N175A的转糖基化的结构特异性。检查了在β-甘露糖残基C-4位置化学修饰的供体的糖基转移,成功获得了糖基化产物。

更新日期:2017-12-04

中文翻译:

供体β-N-乙酰氨基葡萄糖氨基糖苷酶催化的转糖基化反应结构的特异性
关键点:使用非天然四糖衍生物研究了糖基施主对hi.alis (hiemalis)(endo- M)的内-β- N-乙酰氨基葡糖苷酶及其突变体N175Q和N175A的转糖基化的结构特异性。检查了在β-甘露糖残基C-4位置化学修饰的供体的糖基转移,成功获得了糖基化产物。




























 京公网安备 11010802027423号
京公网安备 11010802027423号