当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Benzyne‐Promoted Curtius‐Type Rearrangement of Acyl Hydrazides in the Presence of Nucleophiles
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-16 , DOI: 10.1002/ajoc.201700598 Jing-Yu Guo 1 , Chen-Hao Zhong 1 , Zeng-Yang He 1 , Shi-Kai Tian 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-16 , DOI: 10.1002/ajoc.201700598 Jing-Yu Guo 1 , Chen-Hao Zhong 1 , Zeng-Yang He 1 , Shi-Kai Tian 1
Affiliation
|
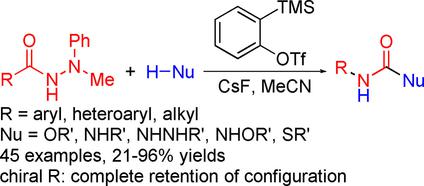
A protocol has been developed for the Curtius‐type rearrangement of acyl hydrazides in the presence of nucleophiles by activating acyl hydrazides with benzyne to generate aminimide intermediates. A wide variety of N′‐methyl‐N′‐phenyl acyl hydrazides were treated with various alcohols in the presence of 2‐(trimethylsilyl)phenyl triflate and CsF under mild conditions to afford structurally diverse carbamates in moderate to excellent yields. Importantly, complete retention of configuration was observed in the reaction of enantioenriched α‐chiral alkanoyl hydrazides. Replacing the alcohol with water, an amine, an N′‐unsubstituted acyl hydrazide, an alkoxyamine, or a thiophenol in the reaction permitted facile synthesis of ureas and analogues.
中文翻译:

亲核试剂存在下苯并促进的乙酰肼的Curtius型重排
已经开发出了一种在亲核试剂存在下通过酰苯与苄炔活化生成酰氨基中间体的方法,用于酰肼的Curtius型重排。各种各样的N' -甲基- ñ ' -苯基酰基酰肼用在2-(三甲基硅烷基)三氟甲磺酸苯基和CsF的温和条件下的存在,得到结构不同的氨基甲酸酯在中度至良好的产率各种醇进行处理。重要的是,在对映体富集的α-手性链烷酰基酰肼的反应中观察到构型的完全保留。在反应中用水代替醇,胺,N'-未取代的酰肼,烷氧基胺或苯硫酚即可轻松合成脲和类似物。
更新日期:2017-11-16
中文翻译:

亲核试剂存在下苯并促进的乙酰肼的Curtius型重排
已经开发出了一种在亲核试剂存在下通过酰苯与苄炔活化生成酰氨基中间体的方法,用于酰肼的Curtius型重排。各种各样的N' -甲基- ñ ' -苯基酰基酰肼用在2-(三甲基硅烷基)三氟甲磺酸苯基和CsF的温和条件下的存在,得到结构不同的氨基甲酸酯在中度至良好的产率各种醇进行处理。重要的是,在对映体富集的α-手性链烷酰基酰肼的反应中观察到构型的完全保留。在反应中用水代替醇,胺,N'-未取代的酰肼,烷氧基胺或苯硫酚即可轻松合成脲和类似物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号