当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Relationship of Propargylamine‐Based HDAC Inhibitors
ChemMedChem ( IF 3.6 ) Pub Date : 2017-11-30 , DOI: 10.1002/cmdc.201700550 Matthias Wünsch 1 , Johanna Senger 2 , Philipp Schultheisz 1 , Sabrina Schwarzbich 1 , Karin Schmidtkunz 2 , Carmela Michalek 1 , Michaela Klaß 1 , Stefanie Goskowitz 1 , Philipp Borchert 1 , Lucas Praetorius 3 , Wolfgang Sippl 3 , Manfred Jung 2 , Norbert Sewald 1
ChemMedChem ( IF 3.6 ) Pub Date : 2017-11-30 , DOI: 10.1002/cmdc.201700550 Matthias Wünsch 1 , Johanna Senger 2 , Philipp Schultheisz 1 , Sabrina Schwarzbich 1 , Karin Schmidtkunz 2 , Carmela Michalek 1 , Michaela Klaß 1 , Stefanie Goskowitz 1 , Philipp Borchert 1 , Lucas Praetorius 3 , Wolfgang Sippl 3 , Manfred Jung 2 , Norbert Sewald 1
Affiliation
|
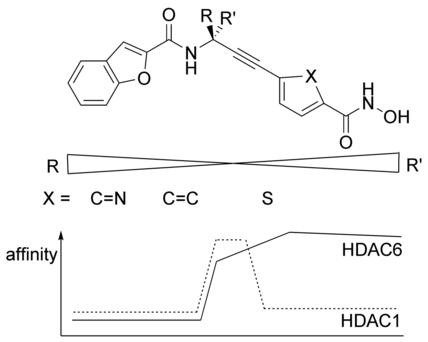
As histone deacetylases (HDACs) play an important role in the treatment of cancer, their selective inhibition has been the subject of various studies. These continuous investigations have given rise to a large collection of pan‐ and selective HDAC inhibitors, containing diverse US Food and Drug Administration (FDA)‐approved representatives. In previous studies, a class of alkyne‐based HDAC inhibitors was presented. We modified this scaffold in two previously neglected regions and compared their cytotoxicity and affinity toward HDAC1, HDAC6, and HDAC8. We were able to show that R‐configured propargylamines contribute to increased selectivity for HDAC6. Docking studies on available HDAC crystal structures were carried out to rationalize the observed selectivity of the compounds. Substitution of the aromatic portion by a thiophene derivative results in high affinity and low cytotoxicity, indicating an improved drug tolerance.
中文翻译:

基于炔丙胺的HDAC抑制剂的结构-活性关系
由于组蛋白脱乙酰基酶(HDACs)在癌症的治疗中起着重要的作用,其选择性抑制已成为各种研究的主题。这些不断的研究引起了广泛的HDAC抑制剂和选择性HDAC抑制剂的收集,其中包括美国食品和药物管理局(FDA)批准的各种代表。在先前的研究中,提出了一类基于炔烃的HDAC抑制剂。我们在两个先前被忽略的区域中修改了该支架,并比较了它们对HDAC1,HDAC6和HDAC8的细胞毒性和亲和力。我们能够证明R配置的炔丙基胺有助于提高对HDAC6的选择性。对可用的HDAC晶体结构进行了对接研究,以合理化所观察到的化合物的选择性。噻吩衍生物取代芳族部分导致高亲和力和低细胞毒性,表明药物耐受性提高。
更新日期:2017-11-30
中文翻译:

基于炔丙胺的HDAC抑制剂的结构-活性关系
由于组蛋白脱乙酰基酶(HDACs)在癌症的治疗中起着重要的作用,其选择性抑制已成为各种研究的主题。这些不断的研究引起了广泛的HDAC抑制剂和选择性HDAC抑制剂的收集,其中包括美国食品和药物管理局(FDA)批准的各种代表。在先前的研究中,提出了一类基于炔烃的HDAC抑制剂。我们在两个先前被忽略的区域中修改了该支架,并比较了它们对HDAC1,HDAC6和HDAC8的细胞毒性和亲和力。我们能够证明R配置的炔丙基胺有助于提高对HDAC6的选择性。对可用的HDAC晶体结构进行了对接研究,以合理化所观察到的化合物的选择性。噻吩衍生物取代芳族部分导致高亲和力和低细胞毒性,表明药物耐受性提高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号