Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of (Benzyl)biphenyls by Successive Suzuki–Miyaura Coupling of Phenylboronic Acids with 4-Bromobenzyl Acetate under Air Atmosphere
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acsomega.7b01450 Masato Ohsumi 1 , Nagatoshi Nishiwaki
ACS Omega ( IF 3.7 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acsomega.7b01450 Masato Ohsumi 1 , Nagatoshi Nishiwaki
Affiliation
|
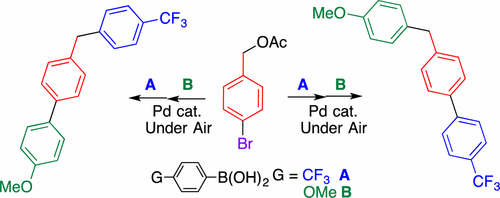
An efficient Pd-catalyzed cross-coupling reaction of phenylboronic acids and benzyl carbonates was developed, producing diarylmethanes. Benzyl acetates could also be used as coupling partners instead of benzyl carbonates, affording diarylmethanes in comparable yields. This reaction can be conducted under air atmosphere without any care for moisture and oxygen. The ester function showed an intermediate reactivity between chloro and bromo groups. This property facilitated the selective synthesis of diverse (benzyl)biphenyls by successive Suzuki–Miyaura coupling reactions using bromo- and chloro-substituted benzyl esters with two types of boronic acids.
中文翻译:

空气中大气中苯硼酸与乙酸4-溴苄酯的连续Suzuki-Miyaura偶联选择性合成(苄基)联苯
开发了一种高效的Pd催化的苯基硼酸与碳酸苄酯的交叉偶联反应,生产出二芳基甲烷。乙酸苄酯也可以代替碳酸苄酯用作偶联伙伴,以可比的收率提供二芳基甲烷。该反应可以在空气气氛下进行而无需任何水分和氧气。酯官能团显示出氯基和溴基之间的中间反应性。该性质通过使用溴和氯取代的苄基酯与两种类型的硼酸的连续Suzuki-Miyaura偶联反应,促进了多种(苄基)联苯的选择性合成。
更新日期:2017-11-09
中文翻译:

空气中大气中苯硼酸与乙酸4-溴苄酯的连续Suzuki-Miyaura偶联选择性合成(苄基)联苯
开发了一种高效的Pd催化的苯基硼酸与碳酸苄酯的交叉偶联反应,生产出二芳基甲烷。乙酸苄酯也可以代替碳酸苄酯用作偶联伙伴,以可比的收率提供二芳基甲烷。该反应可以在空气气氛下进行而无需任何水分和氧气。酯官能团显示出氯基和溴基之间的中间反应性。该性质通过使用溴和氯取代的苄基酯与两种类型的硼酸的连续Suzuki-Miyaura偶联反应,促进了多种(苄基)联苯的选择性合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号