当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A regioselective one-pot aza-Friedel–Crafts reaction for primary, secondary and tertiary anilines using a heterogeneous catalyst
Green Chemistry ( IF 9.8 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7gc02038d Giovanna Bosica 1, 2, 3, 4 , Roderick Abdilla 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7gc02038d Giovanna Bosica 1, 2, 3, 4 , Roderick Abdilla 1, 2, 3, 4
Affiliation
|
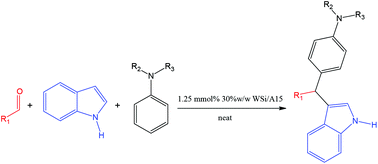
A one-pot multicomponent aza-Friedel–Crafts reaction was performed under green, neat and heterogeneous conditions in the presence of 1.25 mmol% of 30% w/w silicotungstic acid supported on Amberlyst 15 beads. In a yet unprecedented attempt, both primary and secondary anilines could provide the 3-substituted indole product in good to excellent yields usually at room temperature whilst tertiary anilines gave moderate results. Interestingly all reactions proceeded regioselectively via two separate C–C bond forming reactions rather than C–C and C–N bond forming steps. The catalyst is easy, safe and environmentally-benign to prepare, completely recoverable and recyclable up to 5 times.
中文翻译:

使用非均相催化剂对伯,仲和叔苯胺进行区域选择性的一锅氮杂-Friedel-Crafts反应
在绿色,纯净和非均质条件下,在Amberlyst 15磁珠上负载1.25 mmol%的30%w / w硅钨酸的条件下,进行一锅多组分aza-Friedel-Crafts反应。在一项前所未有的尝试中,伯胺和仲苯胺通常都可以在室温下以良好的优异产率提供3-取代的吲哚产物,而叔苯胺给出的结果中等。有趣的是,所有反应都是通过两个单独的C–C键形成反应而不是C–C和C–N键形成步骤进行区域选择性的。该催化剂易于制备,安全且对环境无害,可完全回收和循环使用多达5次。
更新日期:2017-11-09
中文翻译:

使用非均相催化剂对伯,仲和叔苯胺进行区域选择性的一锅氮杂-Friedel-Crafts反应
在绿色,纯净和非均质条件下,在Amberlyst 15磁珠上负载1.25 mmol%的30%w / w硅钨酸的条件下,进行一锅多组分aza-Friedel-Crafts反应。在一项前所未有的尝试中,伯胺和仲苯胺通常都可以在室温下以良好的优异产率提供3-取代的吲哚产物,而叔苯胺给出的结果中等。有趣的是,所有反应都是通过两个单独的C–C键形成反应而不是C–C和C–N键形成步骤进行区域选择性的。该催化剂易于制备,安全且对环境无害,可完全回收和循环使用多达5次。



























 京公网安备 11010802027423号
京公网安备 11010802027423号