PLoS Pathogens ( IF 5.5 ) Pub Date : 2017-11-08 , DOI: 10.1371/journal.ppat.1006714 Isabelle Jupin , Maya Ayach , Lucile Jomat , Sonia Fieulaine , Stéphane Bressanelli
|
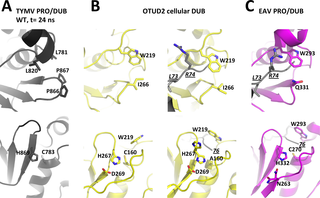
The positive-strand RNA virus Turnip yellow mosaic virus (TYMV) encodes an ovarian tumor (OTU)-like protease/deubiquitinase (PRO/DUB) protein domain involved both in proteolytic processing of the viral polyprotein through its PRO activity, and in removal of ubiquitin chains from ubiquitylated substrates through its DUB activity. Here, the crystal structures of TYMV PRO/DUB mutants and molecular dynamics simulations reveal that an idiosyncratic mobile loop participates in reversibly constricting its unusual catalytic site by adopting "open", "intermediate" or "closed" conformations. The two cis-prolines of the loop form a rigid flap that in the most closed conformation zips up against the other side of the catalytic cleft. The intermediate and closed conformations also correlate with a reordering of the TYMV PRO/DUB catalytic dyad, that then assumes a classical, yet still unusually mobile, OTU DUB alignment. Further structure-based mutants designed to interfere with the loop's mobility were assessed for enzymatic activity in vitro and in vivo, and were shown to display reduced DUB activity while retaining PRO activity. This indicates that control of the switching between the dual PRO/DUB activities resides prominently within this loop next to the active site. Introduction of mutations into the viral genome revealed that the DUB activity contributes to the extent of viral RNA accumulation both in single cells and in whole plants. In addition, the conformation of the mobile flap was also found to influence symptoms severity in planta. Such mutants now provide powerful tools with which to study the specific roles of reversible ubiquitylation in viral infection.
中文翻译:

活性位点附近的移动环充当病毒蛋白酶/去泛素酶双重活性之间的转换
正链RNA病毒芜菁黄花叶病毒(TYMV)编码一个卵巢肿瘤(OTU)样蛋白酶/去泛素酶(PRO / DUB)蛋白质结构域,该结构域通过其PRO活性参与病毒多蛋白的蛋白水解加工,并参与去除遍在蛋白的底物通过其DUB活性产生遍在蛋白链。在这里,TYMV PRO / DUB突变体的晶体结构和分子动力学模拟表明,特异移动环通过采用“开放”,“中间”或“封闭”构象可逆地参与限制其不寻常的催化位点。两个顺式环的脯氨酸形成刚性襟翼,该襟翼在最闭合的构型中紧贴催化裂隙的另一侧。中间构象和闭合构象还与TYMV PRO / DUB催化二重体的重排相关,然后假定其是经典的但仍非常不固定的OTU DUB排列。评估了旨在干扰环移动性的其他基于结构的突变体的体外和体内酶活性,并显示减少了DUB活动,同时保留了PRO活动。这表明对双PRO / DUB活动之间的切换的控制主要位于活动站点旁边的此循环中。将突变引入病毒基因组后发现,DUB活性有助于单细胞和整株植物中病毒RNA的积累。此外,还发现活动瓣的构型影响足底的症状严重程度。现在,此类突变体提供了强大的工具,可用来研究可逆泛素化在病毒感染中的特定作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号