当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Studies of the Glycosidation of 2-O-Substituted 5-Fluorouracil: N-Regioselective Synthesis with the Phase-Transfer-Catalysis Method
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.jpca.7b06602 Yi-Gui Wang 1 , Ericka C. Barnes 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.jpca.7b06602 Yi-Gui Wang 1 , Ericka C. Barnes 1
Affiliation
|
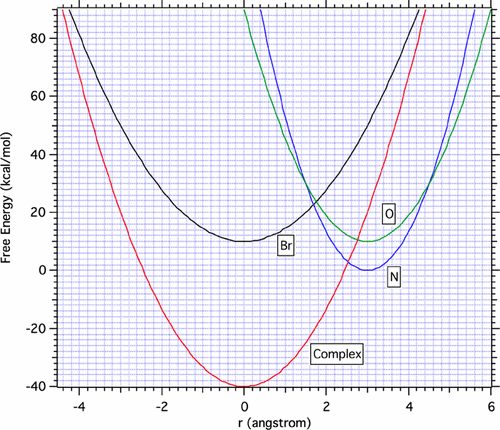
The observed N-regioselective glycosidation of 2-O-substituted 5-fluorouracil (5-FU) via the phase-transfer-catalysis (PTC) method was investigated computationally. The Gibbs free energy reaction barrier of the N-reaction between the 5-FU anion and 1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-α-d-glucopyranose was computed at the MP2/6-311++G(2d,p)//B3LYP/6-31+G* level. The calculated transition states were, in general, quite “loose”, with the ambident reaction sites at the N3- or O4-positions on 5-FU located approximately 2.0 Å from the anomeric carbon. With the SN2 mechanism, the formation of β-glycosides was explained by the characteristics of transition states, and the N-regioselectivity was explained by three considerations: (1) the conformations of initial complexes and the structural requirement of the reactions; (2) the formation of an ionic pair between nBu4N+ and 2-O-substituted 5-FU anions; and (3) the thermodynamic conversion of O-glycosides to N-glycosides. The reactions between the oxocarbenium ion and the 2-O-substituted 5-FU anions (the fast step of SN1 mechanism) were also examined at the same level of theory. Because there were no “promoters” to extract Br in the PTC method, the SN1 mechanism might have an unfavorably high barrier to produce oxocarbenium ion. However, both the formation of β-glycosides and the experimentally observed N-regioselectivity could also be explained by the SN1 mechanism: The former was explained by the neighboring group participation, and the latter was explained by the formation of ionic pairs between nBu4N+ and 2-O-substituted 5-FU anions. The formation of ionic pairs possibly changed the diffusion-controlled mechanism into an activation-controlled mechanism. Two factors were demonstrated by Marcus theory to play an important role for the experimentally observed N-resioselectivity in the PTC method: (1) the thermodynamic stability of N-products over O-products; (2) the formation of ionic pair between nBu4N+ and 2-O-substituted 5-FU anions.
中文翻译:

相转移催化方法2-O取代的5-氟尿嘧啶糖苷化的理论研究:N-区域选择性合成
通过相转移催化(PTC)方法对2 - O-取代的5-氟尿嘧啶(5-FU)的N-区域选择性糖基化进行了计算研究。在MP2上计算了5-FU阴离子与1-溴-1-脱氧-2,3,4,6-四-O-乙酰基-α- d-吡喃葡萄糖之间的N反应的吉布斯自由能反应势垒/ 6-311 ++ G(2d,p)// B3LYP / 6-31 + G *等级。通常,所计算的过渡态非常“松散”,在5-FU的N3-或O4-位置处的环境反应位相对于异头碳约2.0Å。带有S Ñ2机制中,β-糖苷的形成是通过过渡态的特征来解释的,N-区域选择性是通过三个方面的考虑来解释的:(1)初始配合物的构型和反应的结构要求;(2)在n Bu 4 N +和2-O-取代的5-FU阴离子之间形成离子对;(3)O-糖苷向N-糖苷的热力学转化。还以相同的理论水平检查了氧碳鎓离子与2-O-取代的5-FU阴离子之间的反应(S N 1机理的快速步骤)。因为在PTC方法中没有“促进剂”来提取Br,所以S N一种机理可能对产生氧碳en离子具有不利的高阻隔作用。但是,β-糖苷的形成和实验观察到的N-区域选择性也可以通过S N 1机理来解释:前者是通过邻近基团的参与来解释的,后者是通过n之间离子对的形成来解释的。Bu 4 N +和2-O-取代的5-FU阴离子。离子对的形成可能会将扩散控制机制转变为激活控制机制。Marcus理论证明了以下两个因素在PTC方法中对实验观察到的N-重氮选择性起着重要作用:(1)N产物相对于O产物的热力学稳定性;(2)在n Bu 4 N +和2-O-取代的5-FU阴离子之间形成离子对。
更新日期:2017-11-09
中文翻译:

相转移催化方法2-O取代的5-氟尿嘧啶糖苷化的理论研究:N-区域选择性合成
通过相转移催化(PTC)方法对2 - O-取代的5-氟尿嘧啶(5-FU)的N-区域选择性糖基化进行了计算研究。在MP2上计算了5-FU阴离子与1-溴-1-脱氧-2,3,4,6-四-O-乙酰基-α- d-吡喃葡萄糖之间的N反应的吉布斯自由能反应势垒/ 6-311 ++ G(2d,p)// B3LYP / 6-31 + G *等级。通常,所计算的过渡态非常“松散”,在5-FU的N3-或O4-位置处的环境反应位相对于异头碳约2.0Å。带有S Ñ2机制中,β-糖苷的形成是通过过渡态的特征来解释的,N-区域选择性是通过三个方面的考虑来解释的:(1)初始配合物的构型和反应的结构要求;(2)在n Bu 4 N +和2-O-取代的5-FU阴离子之间形成离子对;(3)O-糖苷向N-糖苷的热力学转化。还以相同的理论水平检查了氧碳鎓离子与2-O-取代的5-FU阴离子之间的反应(S N 1机理的快速步骤)。因为在PTC方法中没有“促进剂”来提取Br,所以S N一种机理可能对产生氧碳en离子具有不利的高阻隔作用。但是,β-糖苷的形成和实验观察到的N-区域选择性也可以通过S N 1机理来解释:前者是通过邻近基团的参与来解释的,后者是通过n之间离子对的形成来解释的。Bu 4 N +和2-O-取代的5-FU阴离子。离子对的形成可能会将扩散控制机制转变为激活控制机制。Marcus理论证明了以下两个因素在PTC方法中对实验观察到的N-重氮选择性起着重要作用:(1)N产物相对于O产物的热力学稳定性;(2)在n Bu 4 N +和2-O-取代的5-FU阴离子之间形成离子对。











































 京公网安备 11010802027423号
京公网安备 11010802027423号