当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Calorimetric Study of the Activation of Hydrogen by Tris(pentafluorophenyl)borane and Trimesitylphosphine
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.jpca.7b08582 Adrian Y. Houghton 1 , Tom Autrey 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.jpca.7b08582 Adrian Y. Houghton 1 , Tom Autrey 1
Affiliation
|
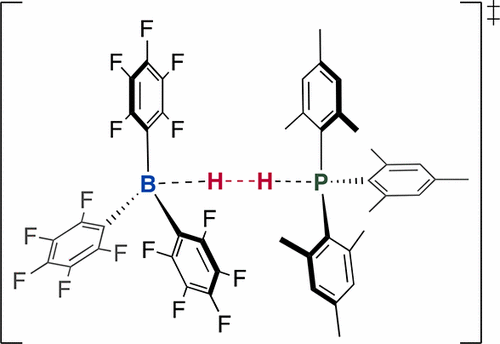
The mechanism of H2 heterolysis by the frustrated Lewis pair of B(C6F5)3 and P(mes)3 was investigated by isothermal reaction calorimetry in the temperature range from 30 to 90 °C. The experimental heat curves were modeled in Berkeley Madonna to obtain both kinetic and thermodynamics data simultaneously. The H2 activation reaction is treated as a single, termolecular step with a rate constant of 0.61 M–2 s–1 at 303 K with an exothermic enthalpy of reaction, ΔHH2 = −141 kJ/mol. An Eyring analysis gave activation parameters of ΔH‡ = 13.6(9) kJ mol–1 and ΔS‡ = −204(85) J K–1 mol–1. Using D2 gas in place of H2 gas provided an opportunity to measure the relative rates of D2 versus H2 heterolysis to yield a the kinetic isotope effect, KIE = 1.1(1).
中文翻译:

三(五氟苯基)硼烷和三甲苯基膦活化氢的量热研究
通过等温反应量热法在30至90°C的温度范围内研究了沮丧的B(C 6 F 5)3和P(mes)3的Lewis对引起的H 2杂化机理。在伯克利·麦当娜(Berkeley Madonna)中对实验热曲线进行建模,以同时获得动力学和热力学数据。为H 2活化反应被视为具有0.61微米的速率常数的单一,三分子步骤-2小号-1在303 K的反应的放热焓,Δ ħ ħ 2 = -141千焦/摩尔。Eyring分析给出了激活参数ΔH ‡= 13.6(9)kJ mol –1和ΔS ‡ = −204(85)JK –1 mol –1。使用D 2气体代替H 2气体提供了一个机会来测量D 2与H 2杂解的相对速率,以产生动力学同位素效应,KIE = 1.1(1)。
更新日期:2017-11-09
中文翻译:

三(五氟苯基)硼烷和三甲苯基膦活化氢的量热研究
通过等温反应量热法在30至90°C的温度范围内研究了沮丧的B(C 6 F 5)3和P(mes)3的Lewis对引起的H 2杂化机理。在伯克利·麦当娜(Berkeley Madonna)中对实验热曲线进行建模,以同时获得动力学和热力学数据。为H 2活化反应被视为具有0.61微米的速率常数的单一,三分子步骤-2小号-1在303 K的反应的放热焓,Δ ħ ħ 2 = -141千焦/摩尔。Eyring分析给出了激活参数ΔH ‡= 13.6(9)kJ mol –1和ΔS ‡ = −204(85)JK –1 mol –1。使用D 2气体代替H 2气体提供了一个机会来测量D 2与H 2杂解的相对速率,以产生动力学同位素效应,KIE = 1.1(1)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号