当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nature and Hierarchy of Noncovalent Interactions in Gas-Phase Binary Complexes of Indole and Benzimidazole with Ethers
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.jpca.7b08627 Aditi Bhattacherjee 1 , Sanjay Wategaonkar 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.jpca.7b08627 Aditi Bhattacherjee 1 , Sanjay Wategaonkar 1
Affiliation
|
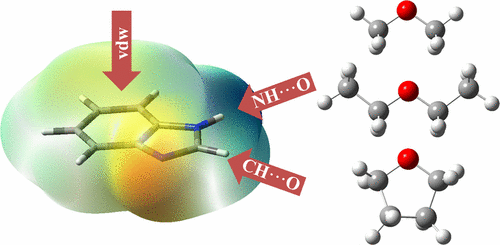
Hierarchy among the weak noncovalent interactions such as van der Waals, electrostatic, hydrogen bonding, etc. dictates the secondary and tertiary structures of proteins as well as their interactions with various ligands. In this work, we investigate the competition between conventional (N–H···O) hydrogen bonds, unconventional (C–H···O) hydrogen bonds, and the van der Waals interaction in the model compounds of the chromophores of the amino acids, tryptophan, and histidine. These include indole (IND), benzimidazole (BIM), and its N-methylated analog (N-methylbenzimidazole, MBIM), which present multiple docking sites. The binary complexes of these molecules with ethers (dimethyl ether, diethyl ether, and tetrahydrofuran), which possess high proton affinity but lack acidic protons (thereby only act as hydrogen bond acceptors), are investigated. The complexes are formed in a supersonic jet and jointly studied by electronic and vibrational spectroscopy as well as quantum chemical calculations. Only the N–H···O bound structures are observed for the complexes of IND and BIM with ethers, although computations predict reasonably competent C–H···O type of structures. Remarkably, IND and BIM produce three (N–H···O) conformers with Me2O but single conformers with Et2O and THF. In the case of MBIM, which lacks a conventional hydrogen bond donor, no evidence for C(2)–H···O hydrogen bonds is seen; instead, the complexes are found to be bound purely by van der Waals interactions. The results indicate that strong N–H···O and even weak van der Waals interactions are thermodynamically favored over C(2)–H···O bound structures in these binary gas-phase complexes.
中文翻译:

吲哚和苯并咪唑与醚的气相二元配合物中非共价相互作用的性质和层次
弱的非共价相互作用(例如范德华力,静电,氢键等)之间的层次决定了蛋白质的二级和三级结构以及它们与各种配体的相互作用。在这项工作中,我们研究了常规(N–H···O)氢键,非常规(C–H···O)氢键和范德华相互作用在生色团模型化合物中的竞争。氨基酸,色氨酸和组氨酸。其中包括吲哚(IND),苯并咪唑(BIM)及其N-甲基化类似物(N-甲基苯并咪唑(MBIM),它具有多个对接位点。研究了这些分子与醚(二甲醚,乙醚和四氢呋喃)的二元配合物,它们具有高质子亲和力但缺乏酸性质子(因此仅充当氢键受体)。络合物在超音速射流中形成,并通过电子和振动光谱以及量子化学计算共同研究。IND和BIM与醚的复合物仅观察到N–H···O结合的结构,尽管计算预测可以合理地胜任C–H···O的结构类型。值得注意的是,IND和BIM与Me 2 O生成三个(NH–··O)构象异构体,而与Et 2生成单个构象子O和THF。对于缺乏常规氢键供体的MBIM,没有发现C(2)–H··O氢键的证据。取而代之的是,发现这些络合物仅受范德华相互作用的束缚。结果表明,在这些二元气相配合物中,强N–H··O甚至弱的范德华相互作用在热力学上优于C(2)–H···O结合结构。
更新日期:2017-11-09
中文翻译:

吲哚和苯并咪唑与醚的气相二元配合物中非共价相互作用的性质和层次
弱的非共价相互作用(例如范德华力,静电,氢键等)之间的层次决定了蛋白质的二级和三级结构以及它们与各种配体的相互作用。在这项工作中,我们研究了常规(N–H···O)氢键,非常规(C–H···O)氢键和范德华相互作用在生色团模型化合物中的竞争。氨基酸,色氨酸和组氨酸。其中包括吲哚(IND),苯并咪唑(BIM)及其N-甲基化类似物(N-甲基苯并咪唑(MBIM),它具有多个对接位点。研究了这些分子与醚(二甲醚,乙醚和四氢呋喃)的二元配合物,它们具有高质子亲和力但缺乏酸性质子(因此仅充当氢键受体)。络合物在超音速射流中形成,并通过电子和振动光谱以及量子化学计算共同研究。IND和BIM与醚的复合物仅观察到N–H···O结合的结构,尽管计算预测可以合理地胜任C–H···O的结构类型。值得注意的是,IND和BIM与Me 2 O生成三个(NH–··O)构象异构体,而与Et 2生成单个构象子O和THF。对于缺乏常规氢键供体的MBIM,没有发现C(2)–H··O氢键的证据。取而代之的是,发现这些络合物仅受范德华相互作用的束缚。结果表明,在这些二元气相配合物中,强N–H··O甚至弱的范德华相互作用在热力学上优于C(2)–H···O结合结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号