当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phellilane L, Sesquiterpene Metabolite of Phellinus linteus: Isolation, Structure Elucidation, and Asymmetric Total Synthesis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.joc.7b02141 Koichiro Ota 1 , Ikuma Yamazaki 1 , Takahiro Saigoku 1 , Mei Fukui 1 , Tomoki Miyata 1 , Kazuo Kamaike 1 , Tatsuya Shirahata 2 , Fumi Mizuno 2 , Yoshihisa Asada 2 , Masao Hirotani 2 , Chieko Ino 2 , Takafumi Yoshikawa 2 , Yoshinori Kobayashi 2 , Hiroaki Miyaoka 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.joc.7b02141 Koichiro Ota 1 , Ikuma Yamazaki 1 , Takahiro Saigoku 1 , Mei Fukui 1 , Tomoki Miyata 1 , Kazuo Kamaike 1 , Tatsuya Shirahata 2 , Fumi Mizuno 2 , Yoshihisa Asada 2 , Masao Hirotani 2 , Chieko Ino 2 , Takafumi Yoshikawa 2 , Yoshinori Kobayashi 2 , Hiroaki Miyaoka 1
Affiliation
|
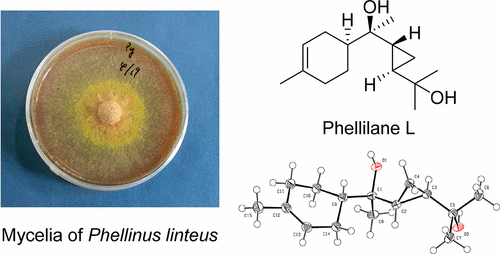
A new cyclopropane-containing sesquiterpenoid, phellilane L (1), was isolated from the medicinal mushroom Phellinus linteus (“Meshimakobu” in Japanese), a member of the Hymenochaetaceae family and a well-known fungus that is widely used in East Asia. The planar structure of 1 was determined on the basis of spectroscopic analysis. The authors achieved the first total synthesis of 1. Our protecting group-free synthesis features a highly stereoselective one-pot synthesis involving an intermolecular alkylation/cyclization/lactonization strategy for construction of the key cyclopropane-γ-lactone intermediate. Additionally, our synthesis determined the absolute configuration of phellilane L (1).
中文翻译:

Phellilane L,桑黄的倍半萜烯代谢产物:分离,结构阐明和不对称的总合成。
一种新的含环丙烷的倍半萜类物质phellilane L(1),是从药用蘑菇桑黄(Phellinus linteus,日语为“ Meshimakobu” )分离而来的,该菌是鬣狗科的成员,也是一种在东亚广泛使用的真菌。的平面结构1被光谱分析的基础上确定的。作者实现的第一全合成1。我们的无保护基合成具有高度立体选择性的一锅合成,涉及分子间烷基化/环化/内酯化策略,用于构建关键的环丙烷-γ-内酯中间体。此外,我们的合成确定了phellilane L(1)的绝对构型。
更新日期:2017-11-09
中文翻译:

Phellilane L,桑黄的倍半萜烯代谢产物:分离,结构阐明和不对称的总合成。
一种新的含环丙烷的倍半萜类物质phellilane L(1),是从药用蘑菇桑黄(Phellinus linteus,日语为“ Meshimakobu” )分离而来的,该菌是鬣狗科的成员,也是一种在东亚广泛使用的真菌。的平面结构1被光谱分析的基础上确定的。作者实现的第一全合成1。我们的无保护基合成具有高度立体选择性的一锅合成,涉及分子间烷基化/环化/内酯化策略,用于构建关键的环丙烷-γ-内酯中间体。此外,我们的合成确定了phellilane L(1)的绝对构型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号