当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-11-09 , DOI: 10.1016/j.chembiol.2017.09.010 Daniel P. Bondeson , Blake E. Smith , George M. Burslem , Alexandru D. Buhimschi , John Hines , Saul Jaime-Figueroa , Jing Wang , Brian D. Hamman , Alexey Ishchenko , Craig M. Crews
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-11-09 , DOI: 10.1016/j.chembiol.2017.09.010 Daniel P. Bondeson , Blake E. Smith , George M. Burslem , Alexandru D. Buhimschi , John Hines , Saul Jaime-Figueroa , Jing Wang , Brian D. Hamman , Alexey Ishchenko , Craig M. Crews
|
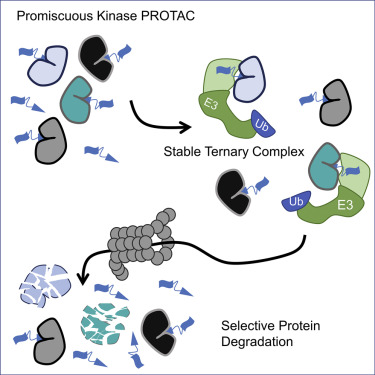
Inhibiting protein function selectively is a major goal of modern drug discovery. Here, we report a previously understudied benefit of small molecule proteolysis-targeting chimeras (PROTACs) that recruit E3 ubiquitin ligases to target proteins for their ubiquitination and subsequent proteasome-mediated degradation. Using promiscuous CRBN- and VHL-recruiting PROTACs that bind >50 kinases, we show that only a subset of bound targets is degraded. The basis of this selectivity relies on protein-protein interactions between the E3 ubiquitin ligase and the target protein, as illustrated by engaged proteins that are not degraded as a result of unstable ternary complexes with PROTAC-recruited E3 ligases. In contrast, weak PROTAC:target protein affinity can be stabilized by high-affinity target:PROTAC:ligase trimer interactions, leading to efficient degradation. This study highlights design guidelines for generating potent PROTACs as well as possibilities for degrading undruggable proteins immune to traditional small-molecule inhibitors.
中文翻译:

PROTAC设计的教训,包括混合弹头的选择性降解
选择性抑制蛋白质功能是现代药物发现的主要目标。在这里,我们报道了以前被研究不足的小分子靶向靶向嵌合体(PROTACs)的益处,该嵌合体募集E3泛素连接酶来靶向蛋白的泛素化和随后的蛋白酶体介导的降解。使用结合> 50种激酶的混杂CRBN和VHL招募的PROTAC,我们显示只有一部分结合的靶标被降解。这种选择性的基础依赖于E3泛素连接酶与靶蛋白之间的蛋白质-蛋白质相互作用,如因与PROTAC招募的E3连接酶不稳定的三元复合物而不会降解的结合蛋白所说明的那样。相比之下,弱的PROTAC:目标蛋白亲和力可以通过高亲和力的target:PROTAC:ligase三聚体相互作用来稳定,导致有效降解。这项研究重点介绍了生成有效PROTAC的设计指南,以及降解对传统小分子抑制剂免疫的不可消耗蛋白质的可能性。
更新日期:2018-01-18
中文翻译:

PROTAC设计的教训,包括混合弹头的选择性降解
选择性抑制蛋白质功能是现代药物发现的主要目标。在这里,我们报道了以前被研究不足的小分子靶向靶向嵌合体(PROTACs)的益处,该嵌合体募集E3泛素连接酶来靶向蛋白的泛素化和随后的蛋白酶体介导的降解。使用结合> 50种激酶的混杂CRBN和VHL招募的PROTAC,我们显示只有一部分结合的靶标被降解。这种选择性的基础依赖于E3泛素连接酶与靶蛋白之间的蛋白质-蛋白质相互作用,如因与PROTAC招募的E3连接酶不稳定的三元复合物而不会降解的结合蛋白所说明的那样。相比之下,弱的PROTAC:目标蛋白亲和力可以通过高亲和力的target:PROTAC:ligase三聚体相互作用来稳定,导致有效降解。这项研究重点介绍了生成有效PROTAC的设计指南,以及降解对传统小分子抑制剂免疫的不可消耗蛋白质的可能性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号