当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Encapsulation and Controlled Release of Protein Guests by the Bacillus subtilis Lumazine Synthase Capsid
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.biochem.7b00669 Xue Han 1 , Kenneth J. Woycechowsky 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.biochem.7b00669 Xue Han 1 , Kenneth J. Woycechowsky 1
Affiliation
|
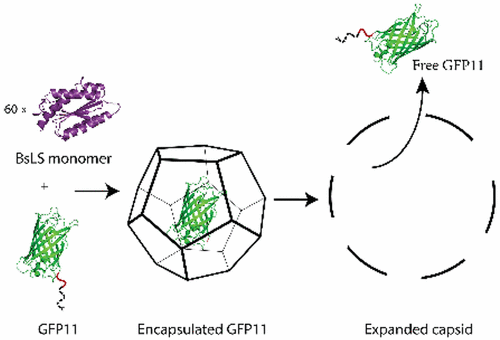
In Bacillus subtilis, the 60-subunit dodecahedral capsid formed by lumazine synthase (BsLS) acts as a container for trimeric riboflavin synthase (BsRS). To test whether the C-terminal sequence of BsRS is responsible for its encapsulation by BsLS, the green fluorescent protein (GFP) was fused to either the last 11 or the last 32 amino acids of BsRS, yielding variant GFP11 or GFP32, respectively. After purification, BsLS capsids that had been co-produced in bacteria with GFP11 and GFP32 are 15- and 6-fold more fluorescent, respectively, than BsLS co-produced with GFP lacking any BsRS fragment, indicating complex formation. Enzyme-linked immunosorbent assay experiments confirm that GFP11 is localized within the BsLS capsid. In addition, fusing the last 11 amino acids of BsRS to the C-terminus of the Abrin A chain also led to its encapsulation by BsLS at a level similar to that of GFP11. Together, these results demonstrate that the C-terminal tail of BsRS can act as an encapsulation tag capable of targeting other proteins to the BsLS capsid interior. As with the natural BsLS–BsRS complex, mild changes in pH and buffer identity trigger dissociation of the GFP11 guest, accompanied by a substantial expansion of the BsLS capsid. This system for protein encapsulation and release provides a novel tool for bionanotechnology.
中文翻译:

枯草芽孢杆菌Lumazine合酶衣壳的蛋白客体的封装和控制释放。
在枯草芽孢杆菌中,由鲁嗪合酶(BsLS)形成的60个亚基十二面体衣壳充当三聚核黄素合酶(BsRS)的容器。为了测试BsRS的C端序列是否负责BsLS的封装,将绿色荧光蛋白(GFP)与BsRS的最后11个或最后32个氨基酸融合,分别产生变体GFP11或GFP32。纯化后,与缺少任何BsRS片段的GFP共同产生的BsLS相比,与GFP11和GFP32共同在细菌中产生的BsLS衣壳的荧光强度分别高15倍和6倍。酶联免疫吸附试验证实GFP11位于BsLS衣壳内。此外,将BsRS的最后11个氨基酸融合到Abrin A链的C末端,也导致BsLS以类似于GFP11的水平将其包封。在一起,这些结果证明BsRS的C末端尾巴可以作为能够将其他蛋白质靶向BsLS衣壳内部的封装标签。与天然BsLS–BsRS复合物一样,pH和缓冲液身份的轻微变化会触发GFP11客体的解离,并伴随BsLS衣壳的显着扩增。该用于蛋白质包封和释放的系统为生物纳米技术提供了一种新颖的工具。pH和缓冲液身份的温和变化会触发GFP11客体的解离,并伴随BsLS衣壳的大幅扩增。该用于蛋白质包封和释放的系统为生物纳米技术提供了一种新颖的工具。pH和缓冲液身份的温和变化会触发GFP11客体的解离,并伴随BsLS衣壳的大幅扩增。该用于蛋白质包封和释放的系统为生物纳米技术提供了一种新颖的工具。
更新日期:2017-11-09
中文翻译:

枯草芽孢杆菌Lumazine合酶衣壳的蛋白客体的封装和控制释放。
在枯草芽孢杆菌中,由鲁嗪合酶(BsLS)形成的60个亚基十二面体衣壳充当三聚核黄素合酶(BsRS)的容器。为了测试BsRS的C端序列是否负责BsLS的封装,将绿色荧光蛋白(GFP)与BsRS的最后11个或最后32个氨基酸融合,分别产生变体GFP11或GFP32。纯化后,与缺少任何BsRS片段的GFP共同产生的BsLS相比,与GFP11和GFP32共同在细菌中产生的BsLS衣壳的荧光强度分别高15倍和6倍。酶联免疫吸附试验证实GFP11位于BsLS衣壳内。此外,将BsRS的最后11个氨基酸融合到Abrin A链的C末端,也导致BsLS以类似于GFP11的水平将其包封。在一起,这些结果证明BsRS的C末端尾巴可以作为能够将其他蛋白质靶向BsLS衣壳内部的封装标签。与天然BsLS–BsRS复合物一样,pH和缓冲液身份的轻微变化会触发GFP11客体的解离,并伴随BsLS衣壳的显着扩增。该用于蛋白质包封和释放的系统为生物纳米技术提供了一种新颖的工具。pH和缓冲液身份的温和变化会触发GFP11客体的解离,并伴随BsLS衣壳的大幅扩增。该用于蛋白质包封和释放的系统为生物纳米技术提供了一种新颖的工具。pH和缓冲液身份的温和变化会触发GFP11客体的解离,并伴随BsLS衣壳的大幅扩增。该用于蛋白质包封和释放的系统为生物纳米技术提供了一种新颖的工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号