当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Colliding-Droplet Microreactor: Rapid On-Demand Inertial Mixing and Metal-Catalyzed Aqueous Phase Oxidation Processes
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.analchem.7b03601 Ryan D. Davis 1 , Michael I. Jacobs 1, 2 , Frances A. Houle 1 , Kevin R. Wilson 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.analchem.7b03601 Ryan D. Davis 1 , Michael I. Jacobs 1, 2 , Frances A. Houle 1 , Kevin R. Wilson 1
Affiliation
|
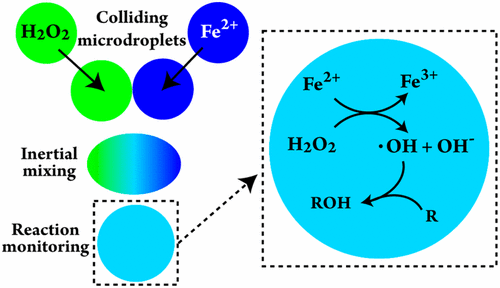
In-depth investigations of the kinetics of aqueous chemistry occurring in microdroplet environments require experimental techniques that allow a reaction to be initiated at a well-defined point in time and space. Merging microdroplets of different reactants is one such approach. The mixing dynamics of unconfined (airborne) microdroplets have yet to be studied in detail, which is an essential step toward widespread use and application of merged droplet microreactors for monitoring chemical reactions. Here, we present an on-demand experimental approach for initiating chemical reactions in and characterizing the mixing dynamics of colliding airborne microdroplets (40 ± 5 μm diameter) using a streak-based fluorescence microscopy technique. The advantages of this approach include the ability to generate two well-controlled monodisperse microdroplet streams and collide (and thus mix) the microdroplets with high spatial and temporal control while consuming small amounts of sample (<0.1 μL/s). Mixing times are influenced not only by the velocity at which microdroplets collide but also the geometry of the collision (i.e., head-on vs off-center collision). For head-on collisions, we achieve submillisecond mixing times ranging from ∼900 μs at a collision velocity of 0.1 m/s to <200 μs at ∼6 m/s. For low-velocity (<1 m/s) off-center collisions, mixing times were consistent with the head-on cases. For high-velocity (i.e., > 1 m/s) off-center collisions, mixing times increased by as much as a factor of 6 (e.g., at ∼6 m/s, mixing times increased from <200 μs for head-on collisions to ∼1200 μs for highly off-center collisions). At collision velocities >7 m/s, droplet separation and fragmentation occurred, resulting in incomplete mixing. These results suggest a limited range of collision velocities over which complete and rapid mixing can be achieved when using airborne merged microdroplets to, e.g., study reaction kinetics when reaction times are short relative to typical bulk reactor mixing times. We benchmark our reactor using an aqueous-phase oxidation reaction: iron-catalyzed hydroxyl radical production from hydrogen peroxide (Fenton’s reaction) and subsequent aqueous-phase oxidation of organic species in solution. Kinetic simulations of our measurements show that quantitative agreement can be obtained using known bulk-phase kinetics for bimolecular reactions in our colliding-droplet microreactor.
中文翻译:

碰撞滴微反应器:快速按需惯性混合和金属催化的水相氧化工艺
对微滴环境中发生的水性化学动力学的深入研究需要实验技术,该技术允许在明确的时间和空间点上引发反应。合并不同反应物的微滴就是这样一种方法。未限制(机载)微滴的混合动力学有待进一步研究,这是迈向为监测化学反应而广泛使用和应用合并的微滴反应器的必不可少的一步。在这里,我们使用基于条纹的荧光显微镜技术,提出了一种按需实验方法,用于启动化学反应并表征碰撞的机载微滴(直径为40±5μm)的混合动力学。这种方法的优点包括能够生成两个控制良好的单分散微滴流,并以高空间和时间控制方式碰撞(并混合)微滴,同时消耗少量样品(<0.1μL/ s)。混合时间不仅受微滴碰撞的速度影响,而且受碰撞几何形状(即正面碰撞与偏心碰撞)的影响。对于正面碰撞,我们实现了从0.1 m / s的碰撞速度到900μs到6 m / s的<200μs的亚毫秒混合时间。对于低速(<1 m / s)偏心碰撞,混合时间与正面情况一致。对于高速(即> 1 m / s)的偏心碰撞,混合时间增加了多达6倍(例如,在〜6 m / s时,混合时间从< 正面碰撞时为200μs,高度偏心碰撞时为约1200μs)。在碰撞速度> 7 m / s时,发生液滴分离和破碎,导致混合不完全。这些结果表明,当使用空中合并的微滴进行研究时,例如,当反应时间相对于典型的本体反应器混合时间短时,研究反应动力学时,碰撞速度的范围有限,可以实现完全和快速的混合。我们使用水相氧化反应对反应器进行基准测试:过氧化氢产生铁催化的羟基自由基(芬顿反应),然后对溶液中的有机物进行水相氧化。
更新日期:2017-11-09
中文翻译:

碰撞滴微反应器:快速按需惯性混合和金属催化的水相氧化工艺
对微滴环境中发生的水性化学动力学的深入研究需要实验技术,该技术允许在明确的时间和空间点上引发反应。合并不同反应物的微滴就是这样一种方法。未限制(机载)微滴的混合动力学有待进一步研究,这是迈向为监测化学反应而广泛使用和应用合并的微滴反应器的必不可少的一步。在这里,我们使用基于条纹的荧光显微镜技术,提出了一种按需实验方法,用于启动化学反应并表征碰撞的机载微滴(直径为40±5μm)的混合动力学。这种方法的优点包括能够生成两个控制良好的单分散微滴流,并以高空间和时间控制方式碰撞(并混合)微滴,同时消耗少量样品(<0.1μL/ s)。混合时间不仅受微滴碰撞的速度影响,而且受碰撞几何形状(即正面碰撞与偏心碰撞)的影响。对于正面碰撞,我们实现了从0.1 m / s的碰撞速度到900μs到6 m / s的<200μs的亚毫秒混合时间。对于低速(<1 m / s)偏心碰撞,混合时间与正面情况一致。对于高速(即> 1 m / s)的偏心碰撞,混合时间增加了多达6倍(例如,在〜6 m / s时,混合时间从< 正面碰撞时为200μs,高度偏心碰撞时为约1200μs)。在碰撞速度> 7 m / s时,发生液滴分离和破碎,导致混合不完全。这些结果表明,当使用空中合并的微滴进行研究时,例如,当反应时间相对于典型的本体反应器混合时间短时,研究反应动力学时,碰撞速度的范围有限,可以实现完全和快速的混合。我们使用水相氧化反应对反应器进行基准测试:过氧化氢产生铁催化的羟基自由基(芬顿反应),然后对溶液中的有机物进行水相氧化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号