当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Evidence Supporting Related Mechanisms for Ru(II)-Catalyzed Dehydrocoupling and Hydrolysis of Amine-Boranes
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acscatal.7b02958 Ainara Telleria 1 , Cristian Vicent 2 , Virginia San Nacianceno 1 , María A. Garralda 1 , Zoraida Freixa 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acscatal.7b02958 Ainara Telleria 1 , Cristian Vicent 2 , Virginia San Nacianceno 1 , María A. Garralda 1 , Zoraida Freixa 1, 3
Affiliation
|
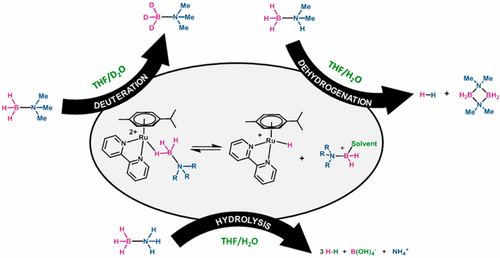
A family of ruthenium(II) half-sandwich complexes was tested for the hydrolytic decomposition of amine-boranes. The analysis of the catalytic results, together with a multilateral approach based on 1H, 11B NMR, and ESI-MS were used to propose a plausible and conceptually unified mechanism for both the hydrolysis and competitive dehydrogenation of amine-boranes. We propose the intermediacy of solvent-stabilized borenium cations during the catalytic cycle, evolving toward dehydrogenation products in distilled THF or releasing amine-hydroxyboranes in aqueous media. Both reaction pathways would liberate up to 1 equivalent of hydrogen through a metal-catalyzed process, but an out-of-cycle low-barrier hydrolysis of amine-hydroxyboranes would produce the 2 additional equivalents of hydrogen in aqueous solutions. Metal-catalyzed deuteration of (non hydrogen-productive) trisubstituted amine-boranes by using D2O as deuterium source was observed, and included as part of the mechanism proposal.
中文翻译:

Ru(II)催化胺-硼烷的脱氢偶联和水解的实验证据支持相关机理
测试了钌(II)半三明治配合物家族对胺-硼烷的水解分解作用。催化结果的分析,以及基于1 H,11的多边方法B NMR和ESI-MS用于为胺-硼烷的水解和竞争性脱氢提出一个合理且概念上统一的机理。我们提出了在催化循环中溶剂稳定的硼阳离子的中间产物,它在蒸馏的THF中趋向于脱氢产物或在水性介质中释放出胺-羟基硼烷。两种反应途径都可以通过金属催化的过程释放多达1当量的氢,但是胺-羟基硼烷的循环外低阻隔水解会在水溶液中产生另外2当量的氢。观察到以D 2 O为氘源的金属催化的(非产氢的)三取代胺-硼烷的氘化反应,并将其作为机理建议的一部分。
更新日期:2017-11-09
中文翻译:

Ru(II)催化胺-硼烷的脱氢偶联和水解的实验证据支持相关机理
测试了钌(II)半三明治配合物家族对胺-硼烷的水解分解作用。催化结果的分析,以及基于1 H,11的多边方法B NMR和ESI-MS用于为胺-硼烷的水解和竞争性脱氢提出一个合理且概念上统一的机理。我们提出了在催化循环中溶剂稳定的硼阳离子的中间产物,它在蒸馏的THF中趋向于脱氢产物或在水性介质中释放出胺-羟基硼烷。两种反应途径都可以通过金属催化的过程释放多达1当量的氢,但是胺-羟基硼烷的循环外低阻隔水解会在水溶液中产生另外2当量的氢。观察到以D 2 O为氘源的金属催化的(非产氢的)三取代胺-硼烷的氘化反应,并将其作为机理建议的一部分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号