当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Robustanoids A and B, two novel pyrrolo[2,3-b]indole alkaloids from Coffea canephora: isolation and total synthesis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1039/c7qo00931c Jianxin Han 1, 2, 3, 4, 5 , Sheng-Tong Niu 1, 2, 3, 4, 5 , Yushuang Liu 1, 2, 3, 4, 5 , Lishe Gan 6, 7, 8, 9 , Tianfu Wang 1, 2, 3, 4, 5 , Chong-Dao Lu 1, 2, 3, 4, 5 , Tao Yuan 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1039/c7qo00931c Jianxin Han 1, 2, 3, 4, 5 , Sheng-Tong Niu 1, 2, 3, 4, 5 , Yushuang Liu 1, 2, 3, 4, 5 , Lishe Gan 6, 7, 8, 9 , Tianfu Wang 1, 2, 3, 4, 5 , Chong-Dao Lu 1, 2, 3, 4, 5 , Tao Yuan 1, 2, 3, 4, 5
Affiliation
|
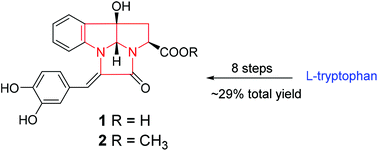
Two novel pyrrolo[2,3-b]indole alkaloids, named robustanoids A (1) and B (2), featuring an unprecedented 1,2,3,4,8b,8c-hexahydro-2a,4a-diazapentaleno[1,6-ab]indene moiety, were isolated from Coffea canephora beans. Their structures were determined by extensive spectroscopic analysis and electronic circular dichroism (ECD) calculation. The total synthesis of compounds 1 and 2 was successfully conducted to give a decent total yield (∼29%) in 8 steps, which also confirmed the structures determined by spectroscopic data. Compound 2 showed α-glucosidase inhibitory activity comparable to that of the positive control, acarbose.
中文翻译:

Robustanoids A和B,两种新的来自咖啡粉的吡咯并[2,3- b ]吲哚生物碱:分离和全合成
两种新颖的吡咯并[2,3- b ]吲哚生物碱,分别称为健壮类动物A(1)和B(2),具有前所未有的1,2,3,4,8b,8c-hexahydro-2a,4a-diazapentaleno [1,从咖啡小豆中分离了6- ab ]茚基部分。通过广泛的光谱分析和电子圆二色性(ECD)计算确定了它们的结构。成功地进行了化合物1和2的全合成,分8步获得了可观的总收率(约29%),这也证实了由光谱数据确定的结构。化合物2显示出与阳性对照阿卡波糖相当的α-葡萄糖苷酶抑制活性。
更新日期:2017-11-08
中文翻译:

Robustanoids A和B,两种新的来自咖啡粉的吡咯并[2,3- b ]吲哚生物碱:分离和全合成
两种新颖的吡咯并[2,3- b ]吲哚生物碱,分别称为健壮类动物A(1)和B(2),具有前所未有的1,2,3,4,8b,8c-hexahydro-2a,4a-diazapentaleno [1,从咖啡小豆中分离了6- ab ]茚基部分。通过广泛的光谱分析和电子圆二色性(ECD)计算确定了它们的结构。成功地进行了化合物1和2的全合成,分8步获得了可观的总收率(约29%),这也证实了由光谱数据确定的结构。化合物2显示出与阳性对照阿卡波糖相当的α-葡萄糖苷酶抑制活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号