当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bond dissociation energy controlled σ-bond metathesis in alkaline-earth-metal hydride catalyzed dehydrocoupling of amines and boranes: a theoretical study
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1039/c7qi00459a Dongdong Xu 1, 2, 3, 4 , Chunhui Shan 1, 2, 3, 4 , Yingzi Li 1, 2, 3, 4 , Xiaotian Qi 1, 2, 3, 4 , Xiaoling Luo 4, 5, 6, 7 , Ruopeng Bai 1, 2, 3, 4 , Yu Lan 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1039/c7qi00459a Dongdong Xu 1, 2, 3, 4 , Chunhui Shan 1, 2, 3, 4 , Yingzi Li 1, 2, 3, 4 , Xiaotian Qi 1, 2, 3, 4 , Xiaoling Luo 4, 5, 6, 7 , Ruopeng Bai 1, 2, 3, 4 , Yu Lan 1, 2, 3, 4
Affiliation
|
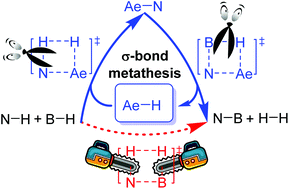
Dehydrocoupling of amines and boranes is an efficient method for the formation of N–B bonds; however, the strong B–H bond dissociation energy (BDE) always restricts non-catalytic reaction pathways. Therefore, alkaline-earth-metal (Ae) hydrides are used as catalysts for this type of reaction because of their lower Ae–H bond energy. A theoretical study was performed to study the mechanism of Ae-catalyzed dehydrocoupling reactions. The computational results show that such reactions are initiated from σ-bond metathesis between Ae hydride catalysts and amines to release molecular hydrogen, followed by borane bonding with amino Ae intermediates. Subsequent hydride transfer yields an amino-borane product and, in the process, regenerates the Ae hydride catalyst. Our theoretical calculations revealed that dehydrogenation is the rate-determining step during σ-bond metathesis in the presence of a magnesium hydride catalyst. We predicted that beryllium hydride could not function as a catalyst because the apparent activation free energy is significantly high. Furthermore, we observed that in calcium or strontium hydride-catalyzed reactions, the rate-limiting step changed to the hydride transfer step. Further density functional theory calculations showed that the BDEs of the Ae–H bond controlled the reactivity of the σ-bond metathesis step.
中文翻译:

碱土金属氢化物催化胺和硼烷的脱氢偶联中键解离能控制的σ键复分解:一个理论研究
胺和硼烷的脱氢偶联是形成N-B键的有效方法。然而,强大的B–H键解离能(BDE)始终限制着非催化反应途径。因此,由于碱土金属(Ae)氢化物具有较低的Ae-H键能,因此被用作此类反应的催化剂。进行了理论研究,以研究Ae催化的脱氢偶联反应的机理。计算结果表明,此类反应是由Ae氢化物催化剂和胺之间的σ键易位引发的,从而释放出分子氢,然后硼烷与氨基Ae中间体键合。随后的氢化物转移产生氨基硼烷产物,并且在该过程中,再生氢化Ae催化剂。我们的理论计算表明,在氢化镁催化剂存在下,脱氢是σ键复分解过程中的决定速率的步骤。我们预测氢化铍不能用作催化剂,因为表观活化自由能非常高。此外,我们观察到在氢化钙或氢化锶反应中,限速步骤变为氢化物转移步骤。进一步的密度泛函理论计算表明,Ae–H键的BDE控制着σ键复分解步骤的反应性。我们观察到在氢化钙或氢化锶反应中,限速步骤变为氢化物转移步骤。进一步的密度泛函理论计算表明,Ae–H键的BDE控制着σ键复分解步骤的反应性。我们观察到在氢化钙或氢化锶反应中,限速步骤变为氢化物转移步骤。进一步的密度泛函理论计算表明,Ae–H键的BDE控制着σ键复分解步骤的反应性。
更新日期:2017-11-08
中文翻译:

碱土金属氢化物催化胺和硼烷的脱氢偶联中键解离能控制的σ键复分解:一个理论研究
胺和硼烷的脱氢偶联是形成N-B键的有效方法。然而,强大的B–H键解离能(BDE)始终限制着非催化反应途径。因此,由于碱土金属(Ae)氢化物具有较低的Ae-H键能,因此被用作此类反应的催化剂。进行了理论研究,以研究Ae催化的脱氢偶联反应的机理。计算结果表明,此类反应是由Ae氢化物催化剂和胺之间的σ键易位引发的,从而释放出分子氢,然后硼烷与氨基Ae中间体键合。随后的氢化物转移产生氨基硼烷产物,并且在该过程中,再生氢化Ae催化剂。我们的理论计算表明,在氢化镁催化剂存在下,脱氢是σ键复分解过程中的决定速率的步骤。我们预测氢化铍不能用作催化剂,因为表观活化自由能非常高。此外,我们观察到在氢化钙或氢化锶反应中,限速步骤变为氢化物转移步骤。进一步的密度泛函理论计算表明,Ae–H键的BDE控制着σ键复分解步骤的反应性。我们观察到在氢化钙或氢化锶反应中,限速步骤变为氢化物转移步骤。进一步的密度泛函理论计算表明,Ae–H键的BDE控制着σ键复分解步骤的反应性。我们观察到在氢化钙或氢化锶反应中,限速步骤变为氢化物转移步骤。进一步的密度泛函理论计算表明,Ae–H键的BDE控制着σ键复分解步骤的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号