当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sustainable synthesis routes towards urazole compounds
Green Chemistry ( IF 9.3 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1039/c7gc02027a Laetitia Vlaminck 1, 2, 3, 4, 5 , Babs Van de Voorde 1, 2, 3, 4, 5 , Filip E. Du Prez 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1039/c7gc02027a Laetitia Vlaminck 1, 2, 3, 4, 5 , Babs Van de Voorde 1, 2, 3, 4, 5 , Filip E. Du Prez 1, 2, 3, 4, 5
Affiliation
|
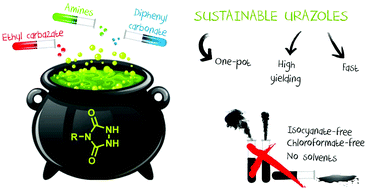
Sustainable synthesis routes towards urazoles, the precursor molecules for triazolinediones, are reported. Not only is the use of isocyanates avoided, but the applied synthetic procedures are also mostly solvent-free, high yielding and performed in an equimolar manner. Two complementary synthesis routes have been developed starting from a wide range of amines, both based on the use of diphenyl carbonate. The first method, which is particularly suitable for the synthesis of bulk urazoles, such as butyl, cyclohexyl or benzyl urazole, is performed in bulk in a one-pot fashion and generally results in yields between 87% and 96%. The second synthesis route, with yields up to 95%, focusses on the implementation of functionalities in the urazole structure and offers the possibility to generate bifunctional urazole compounds.
中文翻译:

合成脲基化合物的可持续合成路线
据报道,向三唑啉二酮的前体分子脲唑的可持续合成途径。不仅避免了异氰酸酯的使用,而且所应用的合成程序也大部分无溶剂,高收率并且以等摩尔方式进行。从广泛的胺开始,已经开发出两种互补的合成路线,两者均基于碳酸二苯酯的使用。第一种方法,特别适合于合成大块脲醛,例如丁基,环己基或苄基脲唑,以单罐方式批量进行,通常收率在87%至96%之间。第二种合成路线的收率高达95%,专注于在urazole结构中实现功能性,并提供了生成双功能urazole化合物的可能性。
更新日期:2017-11-08
中文翻译:

合成脲基化合物的可持续合成路线
据报道,向三唑啉二酮的前体分子脲唑的可持续合成途径。不仅避免了异氰酸酯的使用,而且所应用的合成程序也大部分无溶剂,高收率并且以等摩尔方式进行。从广泛的胺开始,已经开发出两种互补的合成路线,两者均基于碳酸二苯酯的使用。第一种方法,特别适合于合成大块脲醛,例如丁基,环己基或苄基脲唑,以单罐方式批量进行,通常收率在87%至96%之间。第二种合成路线的收率高达95%,专注于在urazole结构中实现功能性,并提供了生成双功能urazole化合物的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号