当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereodivergent asymmetric Michael-alkylation reactions using chiral N,N′-dioxide/metal complexes†
Chemical Science ( IF 7.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1039/c7sc02757e Yulong Kuang 1 , Bin Shen 1 , Li Dai 1 , Qian Yao 1 , Xiaohua Liu 1 , Lili Lin 1 , Xiaoming Feng 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1039/c7sc02757e Yulong Kuang 1 , Bin Shen 1 , Li Dai 1 , Qian Yao 1 , Xiaohua Liu 1 , Lili Lin 1 , Xiaoming Feng 1, 2
Affiliation
|
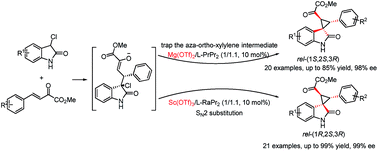
A diastereodivergent asymmetric Michael-alkylation reaction between 3-chloro-oxindoles and β,γ-unsaturated-α-ketoesters has been achieved using L-RaPr2/Sc(OTf)3 and L-PrPr2/Mg(OTf)2 metal complexes as catalysts. Both rel-(1R,2S,3R) and rel-(1S,2S,3R) chiral spiro cyclopropane oxindoles were constructed in good yields, diastereoselectivities and ee values. The diastereodivergent control may originate from different alkylation pathways after the Michael addition, with either intramolecular trapping of the aza-ortho-xylylene intermediate or direct SN2 substitution.
中文翻译:

使用手性 N,N'-二氧化物/金属配合物的非对映发散不对称迈克尔烷基化反应†
使用L-RaPr 2 /Sc(OTf) 3和L-PrPr 2 /Mg(OTf) 2金属配合物实现了 3-氯-羟吲哚和 β,γ-不饱和-α-酮酯之间的非对映发散不对称迈克尔烷基化反应作为催化剂。 rel- ( 1R , 2S , 3R )和rel- ( 1S , 2S , 3R )手性螺环丙烷羟吲哚均具有良好的产率、非对映选择性和ee值。非对映发散控制可能源自迈克尔加成后的不同烷基化途径,通过氮杂邻二甲苯中间体的分子内捕获或直接S N 2 取代。
更新日期:2017-11-08
中文翻译:

使用手性 N,N'-二氧化物/金属配合物的非对映发散不对称迈克尔烷基化反应†
使用L-RaPr 2 /Sc(OTf) 3和L-PrPr 2 /Mg(OTf) 2金属配合物实现了 3-氯-羟吲哚和 β,γ-不饱和-α-酮酯之间的非对映发散不对称迈克尔烷基化反应作为催化剂。 rel- ( 1R , 2S , 3R )和rel- ( 1S , 2S , 3R )手性螺环丙烷羟吲哚均具有良好的产率、非对映选择性和ee值。非对映发散控制可能源自迈克尔加成后的不同烷基化途径,通过氮杂邻二甲苯中间体的分子内捕获或直接S N 2 取代。











































 京公网安备 11010802027423号
京公网安备 11010802027423号