当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective synthesis of gem-diarylalkanes by transition metal-catalyzed asymmetric arylations (TMCAAr)
Chemical Science ( IF 7.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1039/c7sc03404k Tao Jia 1, 2 , Peng Cao 2 , Jian Liao 1, 3
Chemical Science ( IF 7.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1039/c7sc03404k Tao Jia 1, 2 , Peng Cao 2 , Jian Liao 1, 3
Affiliation
|
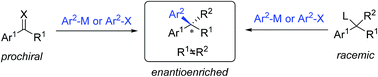
Chiral gem(1,1)-diaryl containing tertiary or quaternary stereogenic centers are present in many natural products and important pharmacophores. While numerous catalytic asymmetric methods enable access to 1,1-diaryl motifs, transition metal-catalyzed asymmetric arylations (TMCAAr) are one of the most powerful methods to prepare enantiopure gem-diarylalkane compounds. The main methodology includes enantioselective 1,2- or 1,4-additions across C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds by arylmetallic reagents; aryl cross-couplings of olefins, benzylic (pseudo)halides and aziridines; asymmetric aryl substitution reactions of allylic substrates; and isotopic benzylic C–H arylation.
C bonds by arylmetallic reagents; aryl cross-couplings of olefins, benzylic (pseudo)halides and aziridines; asymmetric aryl substitution reactions of allylic substrates; and isotopic benzylic C–H arylation.
中文翻译:

过渡金属催化不对称芳基化 (TMCAAr) 对映选择性合成偕二芳基烷烃
手性宝石(1,1)-二芳基含有叔或季立体中心存在于许多天然产物和重要的药效团中。虽然许多催化不对称方法能够获得 1,1-二芳基基序,但过渡金属催化的不对称芳基化 (TMCAAr) 是制备对映体纯宝石-二芳基烷烃化合物的最有效方法之一。主要方法包括通过芳基金属试剂在 C![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O、C
O、C ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N 和 C C 键上进行对映选择性 1,2- 或 1,4-加成;
N 和 C C 键上进行对映选择性 1,2- 或 1,4-加成;![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 烯烃、苄基(伪)卤化物和氮丙啶的芳基交叉偶联;烯丙基底物的不对称芳基取代反应;和同位素苄基 C-H 芳基化。
烯烃、苄基(伪)卤化物和氮丙啶的芳基交叉偶联;烯丙基底物的不对称芳基取代反应;和同位素苄基 C-H 芳基化。
更新日期:2017-11-08
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds by arylmetallic reagents; aryl cross-couplings of olefins, benzylic (pseudo)halides and aziridines; asymmetric aryl substitution reactions of allylic substrates; and isotopic benzylic C–H arylation.
C bonds by arylmetallic reagents; aryl cross-couplings of olefins, benzylic (pseudo)halides and aziridines; asymmetric aryl substitution reactions of allylic substrates; and isotopic benzylic C–H arylation.
中文翻译:

过渡金属催化不对称芳基化 (TMCAAr) 对映选择性合成偕二芳基烷烃
手性宝石(1,1)-二芳基含有叔或季立体中心存在于许多天然产物和重要的药效团中。虽然许多催化不对称方法能够获得 1,1-二芳基基序,但过渡金属催化的不对称芳基化 (TMCAAr) 是制备对映体纯宝石-二芳基烷烃化合物的最有效方法之一。主要方法包括通过芳基金属试剂在 C
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O、C
O、C ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N 和 C C 键上进行对映选择性 1,2- 或 1,4-加成;
N 和 C C 键上进行对映选择性 1,2- 或 1,4-加成;![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 烯烃、苄基(伪)卤化物和氮丙啶的芳基交叉偶联;烯丙基底物的不对称芳基取代反应;和同位素苄基 C-H 芳基化。
烯烃、苄基(伪)卤化物和氮丙啶的芳基交叉偶联;烯丙基底物的不对称芳基取代反应;和同位素苄基 C-H 芳基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号