当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of R–NHC Coupling on the Outcome of R–X Oxidative Addition to Pd/NHC Complexes (R = Me, Ph, Vinyl, Ethynyl)
Organometallics ( IF 2.5 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.organomet.7b00669 Evgeniy G. Gordeev 1 , Dmitry B. Eremin 1 , Victor M. Chernyshev 2 , Valentine P. Ananikov 1
Organometallics ( IF 2.5 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.organomet.7b00669 Evgeniy G. Gordeev 1 , Dmitry B. Eremin 1 , Victor M. Chernyshev 2 , Valentine P. Ananikov 1
Affiliation
|
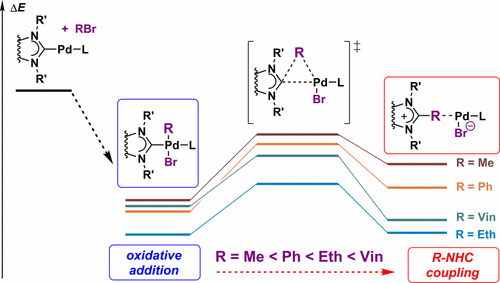
Oxidative addition of organic halides (R–X) to (NHC)Pd0L complexes is involved in numerous metal-catalyzed reactions, and this step is expected to afford (NHC)PdII(R)(X)L intermediate complexes. However, these complexes may undergo further transformation via R–NHC coupling, which removes the NHC ligands from the metal and results in the generation of “bare” NHC-free metal species. The comparative theoretical study carried out in the present work revealed that the kinetic and thermodynamic stability of the (NHC)PdII(R)(X)L oxidative addition intermediates depends strongly on the nature of the organic group R. The predicted reactivity in the R–NHC coupling process decreases in the following order: R = Vinyl > Ethynyl > Ph > Me. Accordingly, for R = Me, a classical (NHC)PdII(R)(X)L intermediate can be expected as a product of the oxidative addition step, whereas for R = Ph, the outcome of the oxidative addition may already contain the NHC-free palladium complex. For R = Ethynyl, comparable amounts of both complexes should be formed, while for R = Vinyl, the NHC-free palladium complex can be the major product of the oxidative addition process. Unusual thermodynamic and kinetic instability of the (NHC)Pd(vinyl)(X)L complex and the tendency to vinyl–NHC coupling predicted by the computational modeling has been confirmed by experimental measurements with online mass spectrometric reaction monitoring. Thus, the outcome of the oxidative addition strongly depends on the type of organic group R and the R–NHC coupling process greatly influences the activity and stability of metal catalysts.
中文翻译:

R–NHC偶联对Pd / NHC配合物(R = Me,Ph,乙烯基,乙炔基)R–X氧化加成结果的影响
有机卤化物(RX)向(NHC)Pd 0 L络合物的氧化加成反应涉及许多金属催化的反应,预计该步骤将提供(NHC)Pd II(R)(X)L中间体络合物。但是,这些络合物可能会通过R–NHC偶联进行进一步转化,这会从金属中除去NHC配体,并导致产生“裸露”的不含NHC的金属物质。在目前的工作中进行的比较理论研究表明(NHC)Pd II的动力学和热力学稳定性(R)(X)L氧化加成中间体在很大程度上取决于有机基团R的性质。R–NHC偶联过程中的预测反应性按以下顺序降低:R =乙烯基>乙炔基> Ph> Me。因此,对于R = Me,经典(NHC)Pd II可以预期(R)(X)L中间体是氧化加成步骤的产物,而对于R = Ph,氧化加成的结果可能已经含有不含NHC的钯配合物。对于R =乙炔基,应形成相当数量的两种络合物,而对于R =乙烯基,不含NHC的钯络合物可能是氧化加成过程的主要产物。(NHC)Pd(乙烯基)(X)L配合物异常的热力学和动力学不稳定以及通过计算模型预测的乙烯基-NHC偶联趋势已通过在线质谱反应监测的实验测量得到证实。因此,氧化加成的结果在很大程度上取决于有机基团R的类型,R–NHC偶联过程极大地影响了金属催化剂的活性和稳定性。
更新日期:2018-02-02
中文翻译:

R–NHC偶联对Pd / NHC配合物(R = Me,Ph,乙烯基,乙炔基)R–X氧化加成结果的影响
有机卤化物(RX)向(NHC)Pd 0 L络合物的氧化加成反应涉及许多金属催化的反应,预计该步骤将提供(NHC)Pd II(R)(X)L中间体络合物。但是,这些络合物可能会通过R–NHC偶联进行进一步转化,这会从金属中除去NHC配体,并导致产生“裸露”的不含NHC的金属物质。在目前的工作中进行的比较理论研究表明(NHC)Pd II的动力学和热力学稳定性(R)(X)L氧化加成中间体在很大程度上取决于有机基团R的性质。R–NHC偶联过程中的预测反应性按以下顺序降低:R =乙烯基>乙炔基> Ph> Me。因此,对于R = Me,经典(NHC)Pd II可以预期(R)(X)L中间体是氧化加成步骤的产物,而对于R = Ph,氧化加成的结果可能已经含有不含NHC的钯配合物。对于R =乙炔基,应形成相当数量的两种络合物,而对于R =乙烯基,不含NHC的钯络合物可能是氧化加成过程的主要产物。(NHC)Pd(乙烯基)(X)L配合物异常的热力学和动力学不稳定以及通过计算模型预测的乙烯基-NHC偶联趋势已通过在线质谱反应监测的实验测量得到证实。因此,氧化加成的结果在很大程度上取决于有机基团R的类型,R–NHC偶联过程极大地影响了金属催化剂的活性和稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号