当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Light-Regulated Synthesis of Cyclic-di-GMP by a Bidomain Construct of the Cyanobacteriochrome Tlr0924 (SesA) without Stable Dimerization
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.biochem.7b00734 Matthew Blain-Hartung 1 , Nathan C. Rockwell 1 , J. Clark Lagarias 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.biochem.7b00734 Matthew Blain-Hartung 1 , Nathan C. Rockwell 1 , J. Clark Lagarias 1
Affiliation
|
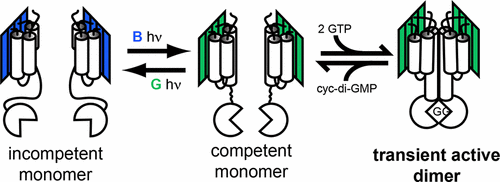
Phytochromes and cyanobacteriochromes (CBCRs) use double-bond photoisomerization of their linear tetrapyrrole (bilin) chromophores within cGMP-specific phosphodiesterases/adenylyl cyclases/FhlA (GAF) domain-containing photosensory modules to regulate activity of C-terminal output domains. CBCRs exhibit photocycles that are much more diverse than those of phytochromes and are often found in large modular proteins such as Tlr0924 (SesA), one of three blue light regulators of cell aggregation in the cyanobacterium Thermosynechococcus elongatus. Tlr0924 contains a single bilin-binding GAF domain adjacent to a C-terminal diguanylate cyclase (GGDEF) domain whose catalytic activity requires formation of a dimeric transition state presumably supported by a multidomain extension at its N-terminus. To probe the structural basis of light-mediated signal propagation from the photosensory input domain to a signaling output domain for a representative CBCR, these studies explore the properties of a bidomain GAF–GGDEF construct of Tlr0924 (Tlr0924Δ) that retains light-regulated diguanylate cyclase activity. Surprisingly, circular dichroism spectroscopy and size exclusion chromatography data do not support formation of stable dimers in either the blue-absorbing 15ZPb dark state or the green-absorbing 15EPg photoproduct state of Tlr0924Δ. Analysis of variants containing site-specific mutations reveals that proper signal transmission requires both chromophorylation of the GAF domain and individual residues within the amphipathic linker region between GAF and GGDEF domains. On the basis of these data, we propose a model in which bilin binding and light signals are propagated from the GAF domain via the linker to alter the equilibrium and interconversion dynamics between active and inactive conformations of the GGDEF domain to favor or disfavor formation of catalytically competent dimers.
中文翻译:

在没有稳定二聚的情况下,由蓝细菌色素Tlr0924(SesA)的双结构域构建体对光调节的环二GMP合成。
植物色素和氰基细菌色素(CBCR)在cGMP特定的磷酸二酯酶/腺苷酸环化酶/ FhlA(GAF)域含光传感模块中使用其线性四吡咯(bilin)生色团的双键光异构化来调节C末端输出域的活性。CBCRs表现出得多比光敏色素的更多样化的,并且通常在大模块化蛋白质如Tlr0924(SESA),在蓝藻细胞聚集的三个蓝色光调节一个发现photocycles细长嗜热。Tlr0924包含与C端双鸟苷酸环化酶(GGDEF)域相邻的单个结合胆碱的GAF域,其催化活性需要形成可能由其N端多域延伸支持的二聚体过渡态。为了探究代表性CBCR从光传感输入域到信号输出域的光介导信号传播的结构基础,这些研究探索了Tlr0924(Tlr0924Δ)的双域GAF–GGDEF构建体的特性,该结构保留了光调节的双鸟苷酸环化酶活动。出人意料的是,圆二色性光谱法和尺寸排阻色谱法数据不支持在任一吸收蓝光的形成稳定的二聚体的15 ż P b暗状态或吸收绿光的15È P克Tlr0924Δ的光化产物的状态。对包含位点特异性突变的变体的分析表明,正确的信号传递既需要GAF域的发色作用,又需要GAF和GGDEF域之间的两亲性接头区域内的单个残基。根据这些数据,我们提出了一个模型,其中Bilin结合和光信号通过接头从GAF结构域传播,以改变GGDEF结构域的活性和非活性构象之间的平衡和相互转化动力学,从而有利于或不利于催化形式的形成。胜任的二聚体。
更新日期:2017-11-08
中文翻译:

在没有稳定二聚的情况下,由蓝细菌色素Tlr0924(SesA)的双结构域构建体对光调节的环二GMP合成。
植物色素和氰基细菌色素(CBCR)在cGMP特定的磷酸二酯酶/腺苷酸环化酶/ FhlA(GAF)域含光传感模块中使用其线性四吡咯(bilin)生色团的双键光异构化来调节C末端输出域的活性。CBCRs表现出得多比光敏色素的更多样化的,并且通常在大模块化蛋白质如Tlr0924(SESA),在蓝藻细胞聚集的三个蓝色光调节一个发现photocycles细长嗜热。Tlr0924包含与C端双鸟苷酸环化酶(GGDEF)域相邻的单个结合胆碱的GAF域,其催化活性需要形成可能由其N端多域延伸支持的二聚体过渡态。为了探究代表性CBCR从光传感输入域到信号输出域的光介导信号传播的结构基础,这些研究探索了Tlr0924(Tlr0924Δ)的双域GAF–GGDEF构建体的特性,该结构保留了光调节的双鸟苷酸环化酶活动。出人意料的是,圆二色性光谱法和尺寸排阻色谱法数据不支持在任一吸收蓝光的形成稳定的二聚体的15 ż P b暗状态或吸收绿光的15È P克Tlr0924Δ的光化产物的状态。对包含位点特异性突变的变体的分析表明,正确的信号传递既需要GAF域的发色作用,又需要GAF和GGDEF域之间的两亲性接头区域内的单个残基。根据这些数据,我们提出了一个模型,其中Bilin结合和光信号通过接头从GAF结构域传播,以改变GGDEF结构域的活性和非活性构象之间的平衡和相互转化动力学,从而有利于或不利于催化形式的形成。胜任的二聚体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号