当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deciphering Nature’s Intricate Way of N,S-Dimethylating l-Cysteine: Sequential Action of Two Bifunctional Adenylation Domains
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.biochem.7b00980 Shogo Mori 1 , Atefeh Garzan 1 , Oleg V. Tsodikov 1 , Sylvie Garneau-Tsodikova 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.biochem.7b00980 Shogo Mori 1 , Atefeh Garzan 1 , Oleg V. Tsodikov 1 , Sylvie Garneau-Tsodikova 1
Affiliation
|
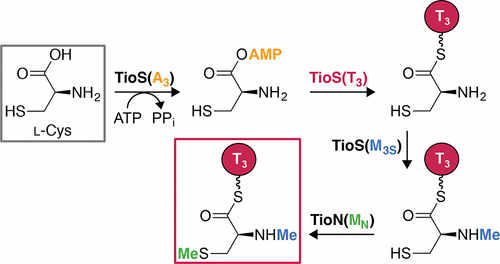
Dimethylation of amino acids consists of an interesting and puzzling series of events that could be achieved, during nonribosomal peptide biosynthesis, either by a single adenylation (A) domain interrupted by a methyltransferase (M) domain or by the sequential action of two of such independent enzymes. Herein, to establish the method by which Nature N,S-dimethylates l-Cys, we studied its formation during thiochondrilline A biosynthesis by evaluating TioS(A3aM3SA3bT3) and TioN(AaMNAb). This study not only led to identification of the exact pathway followed in Nature by these two enzymes for N,S-dimethylation of l-Cys, but also revealed that a single interrupted A domain can N,N-dimethylate amino acids, a novel phenomenon in the nonribosomal peptide field. These findings offer important and useful insights for the development and engineering of novel interrupted A domain enzymes to serve, in the future, as tools for combinatorial biosynthesis.
中文翻译:

破解自然界复杂的N,S-二甲基化半胱氨酸的方式:两个双功能腺苷酸化域的顺序作用
氨基酸的二甲基化包括一系列有趣且令人费解的事件,这些事件可以在非核糖体肽生物合成过程中通过单个甲基化(M)结构域中断的单个腺苷酸化(A)结构域,或通过两个此类独立分子的顺序作用来实现酶。在本文中,为了建立自然N,S-二甲基化L -Cys的方法,我们通过评估TioS(A 3a M 3S A 3b T 3)和TioN(A a M N A b)。这项研究不仅确定了自然界中这两种酶对L -Cys进行N,S-二甲基化所遵循的确切途径,而且还揭示了一个单独的中断A结构域可以使N,N-二甲基化氨基酸,这是一种新现象在非核糖体肽领域。这些发现为新型中断的A结构域酶的开发和工程设计提供了重要而有用的见解,将来可作为组合生物合成的工具。
更新日期:2017-11-07
中文翻译:

破解自然界复杂的N,S-二甲基化半胱氨酸的方式:两个双功能腺苷酸化域的顺序作用
氨基酸的二甲基化包括一系列有趣且令人费解的事件,这些事件可以在非核糖体肽生物合成过程中通过单个甲基化(M)结构域中断的单个腺苷酸化(A)结构域,或通过两个此类独立分子的顺序作用来实现酶。在本文中,为了建立自然N,S-二甲基化L -Cys的方法,我们通过评估TioS(A 3a M 3S A 3b T 3)和TioN(A a M N A b)。这项研究不仅确定了自然界中这两种酶对L -Cys进行N,S-二甲基化所遵循的确切途径,而且还揭示了一个单独的中断A结构域可以使N,N-二甲基化氨基酸,这是一种新现象在非核糖体肽领域。这些发现为新型中断的A结构域酶的开发和工程设计提供了重要而有用的见解,将来可作为组合生物合成的工具。



























 京公网安备 11010802027423号
京公网安备 11010802027423号