当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bimetallic Ru–Ni Catalyzed Aqueous-Phase Guaiacol Hydrogenolysis at Low H2 Pressures
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acscatal.7b02317 Zhicheng Luo 1 , Zhaoxia Zheng 1 , Lei Li 2 , Yi-Tao Cui 3 , Chen Zhao 1, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acscatal.7b02317 Zhicheng Luo 1 , Zhaoxia Zheng 1 , Lei Li 2 , Yi-Tao Cui 3 , Chen Zhao 1, 4
Affiliation
|
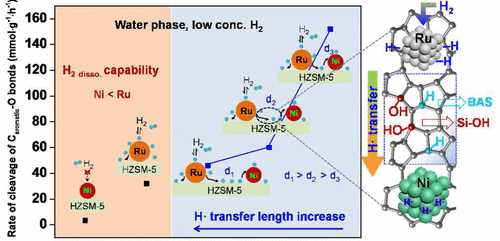
Aqueous-phase hydrogenolysis of renewable biomass at low H2 pressures is an attractive route to selectively produce renewable fuels and valuable chemicals. Here, we show that Ru and Ni nanoparticles (NPs) dispersed on HZSM-5 with an optimum H• radical transfer catalyzed a rapid rate (152 mmol g–1 h–1) of hydrogenolysis of C–O bonds in lignin-derived guaiacol at 240 °C and 2 bar H2 pressure in water. The coimpregnated individual Ru and Ni nanoparticles (NPs) on HZSM-5 were highly dispersed and did not present an alloy structure, but the individual Ru and Ni NPs were in close proximity. The guaiacol hydrogenolysis rates were proportional to the amounts of the adjacent RuO2 and NiO NPs on the calcined samples, suggesting that the closely contacted Ru and Ni NPs on HZSM-5 are the active sites. In the water phase at low H2 pressures, Ru dissociated the hydrogen molecules to H• radicals (H•), and then such radicals were transferred to adjacent Ni atoms to activate the capability of inert Ni centers. The adjustment of the H• transfer length between Ru and Ni NPs led to shorter H• transfer lengths, which resulted in activities as high as 118 mmol g–1 h–1. The transferring and anchoring of H• radicals was considered to be achieved by the Si–OH groups and their defects on HZSM-5, as demonstrated by a temperature-programmed desorption of hydrogen coupled with mass spectroscopy (TPD/H2-MS) experiment. To further shorten the H• transfer length over uniformly formed Ru and Ni nanoparticles, the isolated Ni islands were removed through the incorporation of a Ru precursor that initially occupied the Brønsted acid sites on HZSM-5. By fully activating the two metals in the aqueous phase via an H• transfer mechanism at low H2 pressures, the rational design of bimetallic Ru–Ni catalysts provides a promising approach for achieving substantially high rates in selective hydrogenolysis steps.
中文翻译:

低H 2压力下双金属Ru-Ni催化水相Guaiacol氢解
在低H 2压力下对可再生生物质进行水相氢解是一种有吸引力的途径,可选择性地生产可再生燃料和有价值的化学物质。在这里,我们显示了以最佳H •自由基转移分散在HZSM-5上的Ru和Ni纳米颗粒(NPs)催化了木质素衍生的愈创木酚中C–O键的快速氢解速率(152 mmol g –1 h –1)。在240°C和2 bar H 2的水中压力。HZSM-5上共浸渍的单个Ru和Ni纳米颗粒(NPs)高度分散并且没有合金结构,但是单个Ru和Ni NPs紧密相邻。愈创木酚的氢解速率与相邻RuO 2的量成正比和煅烧样品上的Ni和NP,表明HZSM-5上Ru和Ni的紧密接触是活性位点。在低H 2压力下的水相中,Ru将氢分子解离为H •自由基(H •),然后这些自由基转移到相邻的Ni原子上以激活惰性Ni中心的能力。Ru和Ni NPs之间的H •转移长度的调节导致更短的H •转移长度,从而导致活性高达118 mmol g –1 h –1。H •的转移和锚固自由基被认为是通过Si-OH基团及其在HZSM-5上的缺陷实现的,这是通过程序升温的氢脱附结合质谱(TPD / H 2 -MS)实验证明的。为了进一步缩短均匀形成的Ru和Ni纳米粒子的H •转移长度,通过掺入最初占据HZSM-5上布朗斯台德酸位的Ru前体,可以去除孤立的Ni岛。通过在低H 2压力下通过H •转移机理完全活化水相中的两种金属,合理设计双金属Ru-Ni催化剂提供了一种有希望的方法,可以在选择性氢解步骤中获得很高的产率。
更新日期:2017-11-08
中文翻译:

低H 2压力下双金属Ru-Ni催化水相Guaiacol氢解
在低H 2压力下对可再生生物质进行水相氢解是一种有吸引力的途径,可选择性地生产可再生燃料和有价值的化学物质。在这里,我们显示了以最佳H •自由基转移分散在HZSM-5上的Ru和Ni纳米颗粒(NPs)催化了木质素衍生的愈创木酚中C–O键的快速氢解速率(152 mmol g –1 h –1)。在240°C和2 bar H 2的水中压力。HZSM-5上共浸渍的单个Ru和Ni纳米颗粒(NPs)高度分散并且没有合金结构,但是单个Ru和Ni NPs紧密相邻。愈创木酚的氢解速率与相邻RuO 2的量成正比和煅烧样品上的Ni和NP,表明HZSM-5上Ru和Ni的紧密接触是活性位点。在低H 2压力下的水相中,Ru将氢分子解离为H •自由基(H •),然后这些自由基转移到相邻的Ni原子上以激活惰性Ni中心的能力。Ru和Ni NPs之间的H •转移长度的调节导致更短的H •转移长度,从而导致活性高达118 mmol g –1 h –1。H •的转移和锚固自由基被认为是通过Si-OH基团及其在HZSM-5上的缺陷实现的,这是通过程序升温的氢脱附结合质谱(TPD / H 2 -MS)实验证明的。为了进一步缩短均匀形成的Ru和Ni纳米粒子的H •转移长度,通过掺入最初占据HZSM-5上布朗斯台德酸位的Ru前体,可以去除孤立的Ni岛。通过在低H 2压力下通过H •转移机理完全活化水相中的两种金属,合理设计双金属Ru-Ni催化剂提供了一种有希望的方法,可以在选择性氢解步骤中获得很高的产率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号