当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorbed Hydroxide Does Not Participate in the Volmer Step of Alkaline Hydrogen Electrocatalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acscatal.7b02787 Saad Intikhab 1 , Joshua D. Snyder 1 , Maureen H. Tang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acscatal.7b02787 Saad Intikhab 1 , Joshua D. Snyder 1 , Maureen H. Tang 1
Affiliation
|
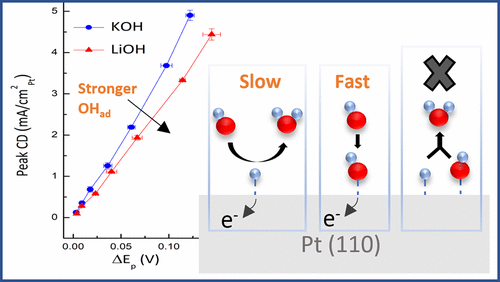
The sluggish kinetics of the alkaline hydrogen electrode have been attributed to the need to adsorb both H and OH optimally. In this work, single-crystal voltammetry and microkinetic modeling show that an OH-mediated mechanism is not viable on Pt(110). Only a direct Volmer step can explain observed kinetic trends with OH adsorption strength in KOH and LiOH electrolytes. Instead, OH behaves as a rapidly equilibrated spectator species that decreases available surface sites and slows hydrogen kinetics. These results identify kinetic barriers from interfacial water structure, not adsorption energies, as key to explaining changes in hydrogen kinetics with pH.
中文翻译:

吸附的氢氧化物不参与碱性氢电催化的沃尔默步骤
碱性氢电极反应迟钝的动力学归因于需要最佳地吸附H和OH。在这项工作中,单晶伏安法和微动力学模型表明,OH介导的机理在Pt(110)上不可行。只有直接的Volmer步骤才能解释在KOH和LiOH电解质中观察到的动力学趋势以及OH的吸附强度。取而代之的是,OH表现为一种快速平衡的观众物质,可减少可用的表面位点并减慢氢动力学。这些结果确定了界面水结构的动力学障碍,而不是吸附能,是解释氢动力学随pH变化的关键。
更新日期:2017-11-08
中文翻译:

吸附的氢氧化物不参与碱性氢电催化的沃尔默步骤
碱性氢电极反应迟钝的动力学归因于需要最佳地吸附H和OH。在这项工作中,单晶伏安法和微动力学模型表明,OH介导的机理在Pt(110)上不可行。只有直接的Volmer步骤才能解释在KOH和LiOH电解质中观察到的动力学趋势以及OH的吸附强度。取而代之的是,OH表现为一种快速平衡的观众物质,可减少可用的表面位点并减慢氢动力学。这些结果确定了界面水结构的动力学障碍,而不是吸附能,是解释氢动力学随pH变化的关键。









































 京公网安备 11010802027423号
京公网安备 11010802027423号