当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Infrared Spectra of the 1-Chloromethyl-1-methylallyl and 1-Chloromethyl-2-methylallyl Radicals Isolated in Solid para-Hydrogen
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.jpca.7b07922 Jay C. Amicangelo,Yuan-Pern Lee
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.jpca.7b07922 Jay C. Amicangelo,Yuan-Pern Lee
|
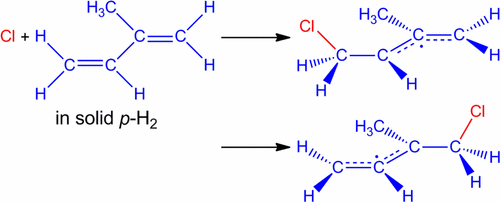
The reaction of chlorine atoms (Cl) with isoprene (2-methyl-1,3-butadiene, C5H8) in solid para-hydrogen (p-H2) matrices at 3.2 K was studied using infrared (IR) spectroscopy. Mixtures of C5H8 and Cl2 were codeposited in p-H2 at 3.2 K, followed by irradiation with ultraviolet light at 365 nm to induce the photodissociation of Cl2 and the subsequent reaction of the Cl atoms with C5H8. Upon 365 nm photolysis, a multitude of new lines appeared in the IR spectrum, and, based on the secondary photolysis behavior, it was determined that the majority of the new lines belong to two distinct chemical species, designated as set A (intense lines at 1237.9, 807.8, and 605.6/608.2 cm–1, and several other weaker lines) and set B (intense lines at 942.4, 1257.7, 796.7/798.5, 667.9, and 569.7 cm–1, and several other weaker lines). Quantum-chemical calculations were performed at the B3PW91/6-311++G(2d,2p) level for ·C5H7 and the four possible isomers of the ·C5H8Cl radicals, produced from the addition of the Cl atom to the four distinct sites of carbon atoms in C5H8, to determine the relative energetics and predict IR spectra for each radical. The newly observed lines of sets A and B are assigned to the 1-chloromethyl-2-methylallyl radical (addition to carbon 4) and the 1-chloromethyl-1-methylallyl radical (addition to carbon 1) according to comparison with predicted IR spectra of possible products. The 1-chloromethyl-2-methylallyl radical and 1-chloromethyl-1-methylallyl radicals were predicted to be the most stable, with the latter ∼8 kJ mol–1 lower in energy than the former. The ratio of the 1-chloromethyl-1-methylallyl to the 1-chloromethyl-2-methylallyl radicals is estimated to be (1.2 ± 0.5):1.0, indicating that the two radicals are produced in approximately equal amounts. The exclusive production of the radicals involving the addition of the Cl atom to the two terminal carbons of isoprene is analogous to what was previously observed for the reaction of Cl atoms with trans-1,3-butadiene in solid p-H2.
中文翻译:

固体对氢中1-氯甲基-1-甲基烯丙基和1-氯甲基-2-甲基烯丙基的红外光谱
使用红外(IR)光谱研究了氯原子(Cl)与异戊二烯(2-甲基-1,3-丁二烯,C 5 H 8)在固体对氢(p -H 2)基质中在3.2 K下的反应。将C 5 H 8和Cl 2的混合物共沉积在3.2 K的p -H 2中,然后在365 nm处用紫外线照射以诱导Cl 2的光解离和随后Cl原子与C 5 H 8的反应。。在365 nm处进行光解后,IR光谱中出现了许多新谱线,并且根据二次光解行为,确定大多数新谱线属于两个不同的化学物种,称为A组( 1237.9、807.8和605.6 / 608.2 cm –1以及其他一些较弱的线),并设置B(强线在942.4、1257.7、796.7 / 798.5、667.9和569.7 cm –1以及其他一些较弱的线)。对C 5 H 7和由加成Cl产生的·C 5 H 8 Cl基的四个可能的异构体,在B3PW91 / 6-311 ++ G(2d,2p)浓度下进行了量子化学计算。原子与C 5中碳原子的四个不同位点H 8,以确定每个自由基的相对能量,并预测IR光谱。根据与预测的IR光谱比较,将新观察到的A和B组谱线分配给1-氯甲基-2-甲基烯丙基(加成碳4)和1-氯甲基-1-甲基烯丙基(加成碳1)可能的产品。预测1-氯甲基-2-甲基烯丙基和1-氯甲基-1-甲基烯丙基是最稳定的,后者约为8 kJ mol –1能量比前者低。1-氯甲基-1-甲基烯丙基与1-氯甲基-2-甲基烯丙基的比例估计为(1.2±0.5):1.0,表明两个自由基的产生量大致相等。涉及将氯原子加到异戊二烯的两个末端碳原子上的自由基的排他性生成,类似于先前在固体p -H 2中氯原子与反式-1,3-丁二烯反应的观察结果。
更新日期:2017-11-08
中文翻译:

固体对氢中1-氯甲基-1-甲基烯丙基和1-氯甲基-2-甲基烯丙基的红外光谱
使用红外(IR)光谱研究了氯原子(Cl)与异戊二烯(2-甲基-1,3-丁二烯,C 5 H 8)在固体对氢(p -H 2)基质中在3.2 K下的反应。将C 5 H 8和Cl 2的混合物共沉积在3.2 K的p -H 2中,然后在365 nm处用紫外线照射以诱导Cl 2的光解离和随后Cl原子与C 5 H 8的反应。。在365 nm处进行光解后,IR光谱中出现了许多新谱线,并且根据二次光解行为,确定大多数新谱线属于两个不同的化学物种,称为A组( 1237.9、807.8和605.6 / 608.2 cm –1以及其他一些较弱的线),并设置B(强线在942.4、1257.7、796.7 / 798.5、667.9和569.7 cm –1以及其他一些较弱的线)。对C 5 H 7和由加成Cl产生的·C 5 H 8 Cl基的四个可能的异构体,在B3PW91 / 6-311 ++ G(2d,2p)浓度下进行了量子化学计算。原子与C 5中碳原子的四个不同位点H 8,以确定每个自由基的相对能量,并预测IR光谱。根据与预测的IR光谱比较,将新观察到的A和B组谱线分配给1-氯甲基-2-甲基烯丙基(加成碳4)和1-氯甲基-1-甲基烯丙基(加成碳1)可能的产品。预测1-氯甲基-2-甲基烯丙基和1-氯甲基-1-甲基烯丙基是最稳定的,后者约为8 kJ mol –1能量比前者低。1-氯甲基-1-甲基烯丙基与1-氯甲基-2-甲基烯丙基的比例估计为(1.2±0.5):1.0,表明两个自由基的产生量大致相等。涉及将氯原子加到异戊二烯的两个末端碳原子上的自由基的排他性生成,类似于先前在固体p -H 2中氯原子与反式-1,3-丁二烯反应的观察结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号