当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Absorption and Fluorescence Features of an Amphiphilic meso-Pyrimidinylcorrole: Experimental Study and Quantum Chemical Calculations
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.jpca.7b08910 Julia Preiß 1, 2 , Felix Herrmann-Westendorf 1, 2 , Thien H. Ngo 3, 4 , Todd Martínez 5, 6 , Benjamin Dietzek 1, 2, 7 , Jonathan P. Hill 4 , Katsuhiko Ariga 4 , Mikalai M. Kruk 8 , Wouter Maes 9 , Martin Presselt 1, 2, 7, 10
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.jpca.7b08910 Julia Preiß 1, 2 , Felix Herrmann-Westendorf 1, 2 , Thien H. Ngo 3, 4 , Todd Martínez 5, 6 , Benjamin Dietzek 1, 2, 7 , Jonathan P. Hill 4 , Katsuhiko Ariga 4 , Mikalai M. Kruk 8 , Wouter Maes 9 , Martin Presselt 1, 2, 7, 10
Affiliation
|
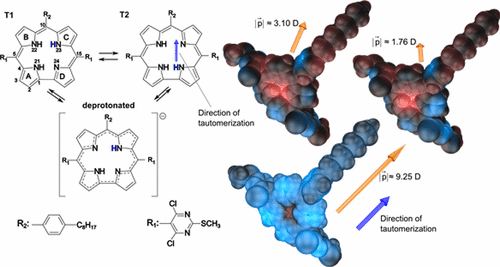
Corroles are emerging as an important class of macrocycles with numerous applications because of their peculiar photophysical and metal chelating properties. meso-Pyrimidinylcorroles are easily deprotonated in certain solvents, which changes their absorption and emission spectra as well as their accessible supramolecular structures. To enable control over the formation of supramolecular structures, the dominant corrole species, i.e., the deprotonated form or one of the two NH-tautomers, needs to be identified. Therefore, we focus in the present article on the determination of the UV–vis spectroscopic properties of the free-base NH-tautomers and the deprotonated form of a new amphiphilic meso-pyrimidinylcorrole that can assemble to supramolecular structures at heterointerfaces as utilized in the Langmuir–Blodgett and liquid–liquid interface precipitation techniques. After quantification of the polarities of the free-base NH-tautomers and the deprotonated form by means of quantum chemically derived electrostatic potential distributions at the corroles’ van der Waals surfaces, the preferential stabilization of (some of) the considered species in solvents of different polarity is identified by means of absorption spectroscopy. For the solutions with complex mixtures of species, we applied fluorescence excitation spectroscopy to estimate the relative weights of the individual corrole species. This technique might also be applied to identify dominating species in molecularly thin films directly on the subphase’ surface of Langmuir–Blodgett troughs. Supported by quantum chemical calculations we were able to differentiate between the spectral signatures of the individual NH-tautomers by means of fluorescence excitation spectroscopy.
中文翻译:

两亲性内消旋嘧啶基甲酚的吸收和荧光特征:实验研究和量子化学计算
由于其独特的光物理和金属螯合特性,它们正逐渐成为一类重要的大环化合物,具有广泛的应用。内消旋-嘧啶基Corroles在某些溶剂中很容易去质子化,这改变了它们的吸收和发射光谱以及可及的超分子结构。为了能够控制超分子结构的形成,需要确定主要的Corrole种类,即去质子化形式或两个NH-互变异构体之一。因此,在本文中,我们重点研究游离碱NH-互变异构体的紫外-可见光谱性质和新两亲性内消旋体的去质子化形式-嘧啶基甲酸酯可在Langmuir-Blodgett和液-液界面沉淀技术中使用,在异质界面组装成超分子结构。在通过在费勒分子的范德华表面上量子化学衍生的静电势分布对游离碱NH-互变异构体和去质子化形式的极性进行定量后,所考虑的物质(某些)在不同溶剂中的优先稳定化极性通过吸收光谱法鉴定。对于具有复杂物种混合物的溶液,我们应用了荧光激发光谱法来估计各个腐蚀物种的相对重量。该技术还可以用于直接在Langmuir-Blodgett槽的子相表面上鉴定分子薄膜中的主要物种。在量子化学计算的支持下,我们能够通过荧光激发光谱法区分各个NH-互变异构体的光谱特征。
更新日期:2017-11-08
中文翻译:

两亲性内消旋嘧啶基甲酚的吸收和荧光特征:实验研究和量子化学计算
由于其独特的光物理和金属螯合特性,它们正逐渐成为一类重要的大环化合物,具有广泛的应用。内消旋-嘧啶基Corroles在某些溶剂中很容易去质子化,这改变了它们的吸收和发射光谱以及可及的超分子结构。为了能够控制超分子结构的形成,需要确定主要的Corrole种类,即去质子化形式或两个NH-互变异构体之一。因此,在本文中,我们重点研究游离碱NH-互变异构体的紫外-可见光谱性质和新两亲性内消旋体的去质子化形式-嘧啶基甲酸酯可在Langmuir-Blodgett和液-液界面沉淀技术中使用,在异质界面组装成超分子结构。在通过在费勒分子的范德华表面上量子化学衍生的静电势分布对游离碱NH-互变异构体和去质子化形式的极性进行定量后,所考虑的物质(某些)在不同溶剂中的优先稳定化极性通过吸收光谱法鉴定。对于具有复杂物种混合物的溶液,我们应用了荧光激发光谱法来估计各个腐蚀物种的相对重量。该技术还可以用于直接在Langmuir-Blodgett槽的子相表面上鉴定分子薄膜中的主要物种。在量子化学计算的支持下,我们能够通过荧光激发光谱法区分各个NH-互变异构体的光谱特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号