当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NMR Study of Solvation Effect on the Geometry of Proton-Bound Homodimers of Increasing Size
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.jpca.7b09285 Andrei A. Gurinov 1, 2 , Gleb S. Denisov 3 , Alexandra O. Borissova 4 , Alexander S. Goloveshkin 4 , Julian Greindl 5 , Hans-Heinrich Limbach 1 , Ilya G. Shenderovich 1, 5
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.jpca.7b09285 Andrei A. Gurinov 1, 2 , Gleb S. Denisov 3 , Alexandra O. Borissova 4 , Alexander S. Goloveshkin 4 , Julian Greindl 5 , Hans-Heinrich Limbach 1 , Ilya G. Shenderovich 1, 5
Affiliation
|
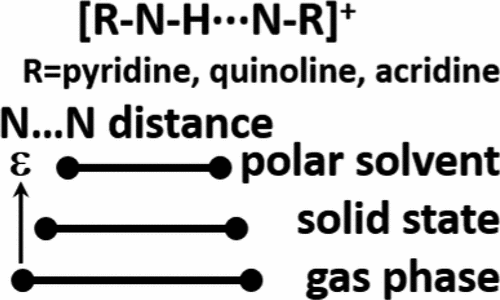
Hydrogen bond geometries in the proton-bound homodimers of quinoline and acridine derivatives in an aprotic polar solution have been experimentally studied using 1H NMR at 120 K. The reported results show that an increase of the dielectric permittivity of the medium results in contraction of the N···N distance. The degree of contraction depends on the homodimer’s size and its substituent-specific solvation features. Neither of these effects can be reproduced using conventional implicit solvent models employed in computational studies. In general, the N···N distance in the homodimers of pyridine, quinoline, and acridine derivatives decreases in the sequence gas phase > solid state > polar solvent.
中文翻译:

溶剂化对尺寸增大的质子束缚均聚物几何构型的核磁共振研究
非质子极性溶液中喹啉和a啶衍生物的质子结合均二聚体中的氢键几何结构已在120 K下使用1 H NMR进行了实验研究。 N···N距离。收缩程度取决于同型二聚体的大小及其取代基特异的溶剂化特征。使用计算研究中使用的常规隐式溶剂模型无法重现这些影响。通常,吡啶,喹啉和a啶衍生物的同二聚体中的N··N距离在气相>固态>极性溶剂的顺序中减小。
更新日期:2017-11-08
中文翻译:

溶剂化对尺寸增大的质子束缚均聚物几何构型的核磁共振研究
非质子极性溶液中喹啉和a啶衍生物的质子结合均二聚体中的氢键几何结构已在120 K下使用1 H NMR进行了实验研究。 N···N距离。收缩程度取决于同型二聚体的大小及其取代基特异的溶剂化特征。使用计算研究中使用的常规隐式溶剂模型无法重现这些影响。通常,吡啶,喹啉和a啶衍生物的同二聚体中的N··N距离在气相>固态>极性溶剂的顺序中减小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号