当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Support for a Dioxyallyl Cation in the Mechanism Leading to (−)-Levoglucosenone
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.joc.7b02109 Ben W. Greatrex 1 , Jan Meisner 2 , Stephen A. Glover 1 , Warwick Raverty 3
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.joc.7b02109 Ben W. Greatrex 1 , Jan Meisner 2 , Stephen A. Glover 1 , Warwick Raverty 3
Affiliation
|
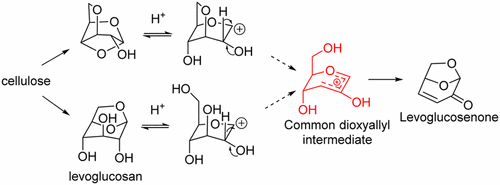
Levoglucosenone (LGO) is the major product formed when cellulose is pyrolyzed in the presence of acid at temperatures between 170 and 350 °C. The current intense interest in biomass conversion has led to a number of reports on its preparation; however, there is still uncertainty on the mechanism leading to LGO. We propose a new mechanism which involves a C2–C1 hydride shift followed by intramolecular trapping of a dioxyallyl cation. The reaction has been modeled using DFT calculations from the known LGO precursors levoglucosan and 1,4:3,6-dianhydro-α-D-glucopyranose to a common intermediate with calculated barriers of 10.6 and 13.5 kcal·mol–1, respectively. A discussion of the literature on the formation of LGO from late pathway intermediates is also provided.
中文翻译:

在导致(-)-左旋葡糖醛酮的机制中对二氧基烯丙基阳离子的支持
左葡萄糖葡酮(LGO)是在酸存在下于170至350°C之间的温度下将纤维素热解时形成的主要产物。当前对生物质转化的浓厚兴趣导致了许多有关其制备的报道。但是,导致LGO的机制仍然不确定。我们提出了一种新的机制,涉及到C2-C1氢化物转移,然后分子内捕获二氧烯丙基阳离子。该反应已通过DFT计算从已知的LGO前体左旋葡聚糖和1,4:3,6-二脱水-α-D-吡喃葡萄糖到一个通用中间体的模拟模型中计算得到,该中间体的阻隔分别为10.6和13.5 kcal·mol –1。还提供了有关从晚期途径中间体形成LGO的文献的讨论。
更新日期:2017-11-08
中文翻译:

在导致(-)-左旋葡糖醛酮的机制中对二氧基烯丙基阳离子的支持
左葡萄糖葡酮(LGO)是在酸存在下于170至350°C之间的温度下将纤维素热解时形成的主要产物。当前对生物质转化的浓厚兴趣导致了许多有关其制备的报道。但是,导致LGO的机制仍然不确定。我们提出了一种新的机制,涉及到C2-C1氢化物转移,然后分子内捕获二氧烯丙基阳离子。该反应已通过DFT计算从已知的LGO前体左旋葡聚糖和1,4:3,6-二脱水-α-D-吡喃葡萄糖到一个通用中间体的模拟模型中计算得到,该中间体的阻隔分别为10.6和13.5 kcal·mol –1。还提供了有关从晚期途径中间体形成LGO的文献的讨论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号