当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
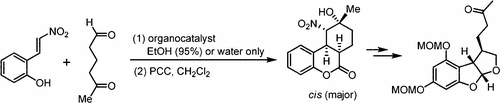
Organocatalytic Enantioselective Michael–Acetalization–Henry Reaction Cascade of 2-Hydroxynitrostyrene and 5-Oxohexanal for the Entry to the Hexahydro-6H-benzo[c]chromenones with Four Consecutive Stereogenic Centers and an Approach to Aflatoxin Analogues
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02178 Yu-You Hsieh,Arun Raja,Bor-Cherng Hong,Prakash Kotame,Wan-Chen Chang,Gene-Hsiang Lee
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02178 Yu-You Hsieh,Arun Raja,Bor-Cherng Hong,Prakash Kotame,Wan-Chen Chang,Gene-Hsiang Lee
|
A domino reaction with the organocatalytic enantioselective Michael–acetalization–Henry reaction of 2-hydroxynitrostyrene and 5-oxohexanal was developed for the synthesis of hexahydro-6H-benzo[c]chromenones with four consecutive stereogenic centers and high enantioselectivity (up to >99% ee). The transformation of a NaBH4-reduced adduct to the aflatoxin system via the Nef-cyclization process was achieved by the assistant of ZnBr2.
中文翻译:

2-羟基硝基苯乙烯和5-氧己醛进入具有四个连续立体中心的六氢-6 H-苯并[ c ]色酮的有机催化对映选择性迈克尔-乙酰化-亨利反应级联和一种黄曲霉毒素类似物的方法
已开发出一种具有2-羟基硝基苯乙烯和5-氧己醛的有机催化对映选择性迈克尔-缩醛化-亨利反应的多米诺反应,用于合成具有四个连续立体中心和高对映选择性(最高> 99的六氢-6 H-苯并[ c ]色酮)。%ee)。通过ZnBr 2的辅助,通过Nef环化过程将NaBH 4还原的加合物转化为黄曲霉毒素体系。
更新日期:2017-11-08
中文翻译:

2-羟基硝基苯乙烯和5-氧己醛进入具有四个连续立体中心的六氢-6 H-苯并[ c ]色酮的有机催化对映选择性迈克尔-乙酰化-亨利反应级联和一种黄曲霉毒素类似物的方法
已开发出一种具有2-羟基硝基苯乙烯和5-氧己醛的有机催化对映选择性迈克尔-缩醛化-亨利反应的多米诺反应,用于合成具有四个连续立体中心和高对映选择性(最高> 99的六氢-6 H-苯并[ c ]色酮)。%ee)。通过ZnBr 2的辅助,通过Nef环化过程将NaBH 4还原的加合物转化为黄曲霉毒素体系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号