当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed sp2 C–H Acetoxylation of N-Aryl Azaindoles/N-Heteroaryl Indolines
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02203 Aniket Mishra 1 , Tripta Kumari Vats 1 , Mahesh P. Nair 1 , Arindam Das 1 , Indubhusan Deb 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02203 Aniket Mishra 1 , Tripta Kumari Vats 1 , Mahesh P. Nair 1 , Arindam Das 1 , Indubhusan Deb 1
Affiliation
|
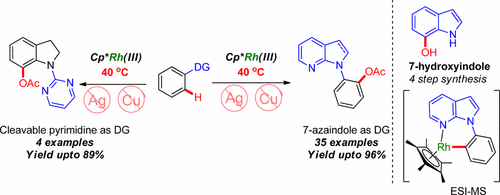
A silver- and copper-free rhodium-catalyzed C–H acetoxylation reaction of azaindoles has been achieved at near ambient temperature employing PIDA as a nonmetallic acetoxy source. The method is highly selective, efficient, and scalable and requires acetic anhydride as the sole additive. The scope of the reaction has been successfully tested with a wide array of medicinally important heterocyclic scaffolds with diverse functional group tolerance. A series of kinetic experiments was conducted to gain detailed insight into the reaction mechanism. The methodology developed could be successfully expanded for C7-acetoxylation of indoline derivatives using pyrimidine as a detachable directing group for the synthesis of 7-hydroxyindole.
中文翻译:

铑催化的N-芳基氮杂吲哚/ N-杂芳基二氢吲哚的sp 2 C–H乙酰氧基化
使用PIDA作为非金属乙酰氧基源,已在接近环境温度的条件下实现了氮杂吲哚的无银和铜铑铑C-H乙酰氧基化反应。该方法是高度选择性的,有效的和可扩展的,并且需要乙酸酐作为唯一的添加剂。该反应的范围已成功地用具有不同官能团耐受性的多种医学上重要的杂环支架进行了测试。进行了一系列动力学实验,以深入了解反应机理。所开发的方法可以成功地扩展为吲哚啉衍生物的C7-乙酰氧基化反应,使用嘧啶作为可分离的7-羟基吲哚合成的导向基团。
更新日期:2017-11-08
中文翻译:

铑催化的N-芳基氮杂吲哚/ N-杂芳基二氢吲哚的sp 2 C–H乙酰氧基化
使用PIDA作为非金属乙酰氧基源,已在接近环境温度的条件下实现了氮杂吲哚的无银和铜铑铑C-H乙酰氧基化反应。该方法是高度选择性的,有效的和可扩展的,并且需要乙酸酐作为唯一的添加剂。该反应的范围已成功地用具有不同官能团耐受性的多种医学上重要的杂环支架进行了测试。进行了一系列动力学实验,以深入了解反应机理。所开发的方法可以成功地扩展为吲哚啉衍生物的C7-乙酰氧基化反应,使用嘧啶作为可分离的7-羟基吲哚合成的导向基团。



























 京公网安备 11010802027423号
京公网安备 11010802027423号