当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiospecific Semisynthesis of Puupehedione-Type Marine Natural Products
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02413 Hong-Shuang Wang 1 , Hui-Jing Li 1 , Xiang Nan 1 , Yuan-Yuan Luo 1 , Yan-Chao Wu 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.joc.7b02413 Hong-Shuang Wang 1 , Hui-Jing Li 1 , Xiang Nan 1 , Yuan-Yuan Luo 1 , Yan-Chao Wu 1, 2
Affiliation
|
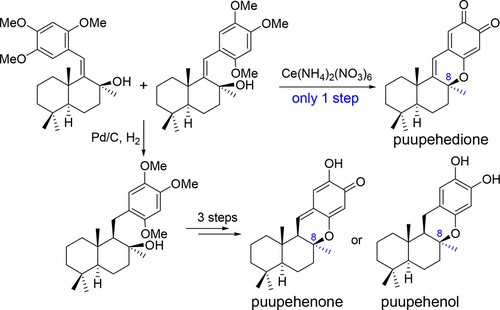
An enantiospecific semisynthesis of puupehedione was achieved from sclareolide in only 7 steps with an overall yield of 25%. The key drimanal trimethoxystyrene skeleton was constructed by the palladium-catalyzed cross-coupling reaction of an aryl iodine and a drimanal hydrazone. An in situ CAN-oxidation/intramolecular oxa-Stork–Danheiser transposition tandem reaction was used as a powerful tool to install concurrently the C and D rings of puupehedione in a one-pot fashion. Its applicability was also showcased by the semisynthesis of puupehenone and puupehenol.
中文翻译:

对苯二酚型海洋天然产物的对映体半合成
从香紫苏内酯仅7个步骤就可完成对映体半合成的puupehedione,总收率为25%。关键的drimanal三甲氧基苯乙烯骨架是通过芳基碘和drimanal hydr的钯催化交叉偶联反应构建的。原位CAN氧化/分子内oxa-Stork–Danheiser转座串联反应被用作一种强大的工具,可以一锅法同时安装puupehedione的C和D环。puupehenone和puupehenol的半合成也证明了其适用性。
更新日期:2017-11-08
中文翻译:

对苯二酚型海洋天然产物的对映体半合成
从香紫苏内酯仅7个步骤就可完成对映体半合成的puupehedione,总收率为25%。关键的drimanal三甲氧基苯乙烯骨架是通过芳基碘和drimanal hydr的钯催化交叉偶联反应构建的。原位CAN氧化/分子内oxa-Stork–Danheiser转座串联反应被用作一种强大的工具,可以一锅法同时安装puupehedione的C和D环。puupehenone和puupehenol的半合成也证明了其适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号