当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, biological evaluation, and docking study of 4‐isochromanone hybrids bearing N‐benzyl pyridinium moiety as dual binding site acetylcholinesterase inhibitors (part II)
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-12-01 , DOI: 10.1111/cbdd.13136 Jia Wang 1 , Chaolei Wang 1 , Zheng Wu 1 , Xinnan Li 1 , Shengtao Xu 1 , Jie Liu 2 , Qinying Lan 3 , Zheying Zhu 4 , Jinyi Xu 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-12-01 , DOI: 10.1111/cbdd.13136 Jia Wang 1 , Chaolei Wang 1 , Zheng Wu 1 , Xinnan Li 1 , Shengtao Xu 1 , Jie Liu 2 , Qinying Lan 3 , Zheying Zhu 4 , Jinyi Xu 1
Affiliation
|
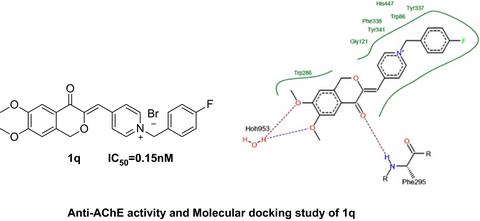
A series of novel 4‐isochromanone compounds bearing N‐benzyl pyridinium moiety were designed and synthesized as acetylcholinesterase (AChE) inhibitors. The biological evaluation showed that most of the target compounds exhibited potent inhibitory activities against AChE. Among them, compound 1q possessed the strongest anti‐AChE activity with an IC50 value of 0.15 nm and high AChE/BuChE selectivity (SI > 5,000). Moreover, compound 1q had low toxicity in normal nerve cells and was relatively stable in rat plasma. Together, the current finding may provide a new approach for the discovery of novel anti‐Alzheimer's disease agents.
中文翻译:

设计,合成,生物学评估和对接带有N-苄基吡啶鎓部分作为双结合位点乙酰胆碱酯酶抑制剂的4-异苯并二氢吡喃酮对接研究(第二部分)
设计并合成了一系列带有N-苄基吡啶鎓部分的新型4-异苯并二氢吡喃酮化合物,作为乙酰胆碱酯酶(AChE)抑制剂。生物学评估表明,大多数目标化合物均显示出对AChE的有效抑制活性。其中,化合物1q具有最强的抗AChE活性,IC 50值为0.15 n m,AChE / BuChE选择性高(SI> 5,000)。此外,化合物1q在正常神经细胞中毒性低,在大鼠血浆中相对稳定。总之,当前的发现可能为发现新型抗阿尔茨海默氏病病原体提供一种新方法。
更新日期:2017-12-01
中文翻译:

设计,合成,生物学评估和对接带有N-苄基吡啶鎓部分作为双结合位点乙酰胆碱酯酶抑制剂的4-异苯并二氢吡喃酮对接研究(第二部分)
设计并合成了一系列带有N-苄基吡啶鎓部分的新型4-异苯并二氢吡喃酮化合物,作为乙酰胆碱酯酶(AChE)抑制剂。生物学评估表明,大多数目标化合物均显示出对AChE的有效抑制活性。其中,化合物1q具有最强的抗AChE活性,IC 50值为0.15 n m,AChE / BuChE选择性高(SI> 5,000)。此外,化合物1q在正常神经细胞中毒性低,在大鼠血浆中相对稳定。总之,当前的发现可能为发现新型抗阿尔茨海默氏病病原体提供一种新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号