当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AgSbF6‐Catalyzed Tandem Reaction of 2‐Alkynylanilines with Cyclic Enynones: Efficient access to 3‐Furo[3,2‐c]chromenylindoles and Related Scaffolds
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-17 , DOI: 10.1002/ajoc.201700511 Anuradha Dagar 1 , Soumitra Guin 1 , Sampak Samanta 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-17 , DOI: 10.1002/ajoc.201700511 Anuradha Dagar 1 , Soumitra Guin 1 , Sampak Samanta 1
Affiliation
|
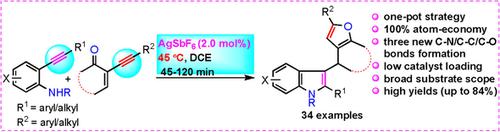
A simple, mild, catalytic and efficient method for the straightforward synthesis of an interesting class of a range of 2‐aryl/alkyl‐substituted‐3‐(2‐aryl/alkyl‐4H‐furo[3,2‐c]chromen‐4‐yl)‐1H‐indoles in good to high yields is reported for the first time. This atom‐efficient method proceeds via AgSbF6‐catalyzed one‐pot sequential intramolecular hydroamination (C−N bond formation) of 2‐alkynylanilines followed by Friedel–Crafts alkylation/oxa‐cyclization (creation of new C−C and C−O bonds) reaction between in situ generated 2‐substituted indoles and several cyclic enynones.
中文翻译:

AgSbF6催化的2-炔基苯胺与环烯酮的串联反应:有效地获得3-Furo [3,2-c]苯并吲哚及其相关骨架
一种简单,温和,催化和有效的方法,用于直接合成一系列有趣的2-芳基/烷基取代的3--3-(2-芳基/烷基-4 H-呋喃[3,2- c ]铬烯首次报道了高产到高产的-4-烷基)-1 H吲哚。通过AgSbF 6催化的2-炔基苯胺单锅顺序分子内加氢胺化反应(C-N键形成),然后进行Friedel-Crafts烷基化/氧杂环化(新的C-C和C-O键的形成) )原位生成的2个取代的吲哚与几个环状烯酮之间的反应。
更新日期:2017-11-17
中文翻译:

AgSbF6催化的2-炔基苯胺与环烯酮的串联反应:有效地获得3-Furo [3,2-c]苯并吲哚及其相关骨架
一种简单,温和,催化和有效的方法,用于直接合成一系列有趣的2-芳基/烷基取代的3--3-(2-芳基/烷基-4 H-呋喃[3,2- c ]铬烯首次报道了高产到高产的-4-烷基)-1 H吲哚。通过AgSbF 6催化的2-炔基苯胺单锅顺序分子内加氢胺化反应(C-N键形成),然后进行Friedel-Crafts烷基化/氧杂环化(新的C-C和C-O键的形成) )原位生成的2个取代的吲哚与几个环状烯酮之间的反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号